Recently, Kai Li’s laboratory at the Department of Biomedical Engineering of the Southern University of Science and Technology (SUSTech) has made significant experimental progress in tumor photodynamic immunotherapy. The research paper, entitled “Acceptor Engineering for Optimized ROS Generation Facilitates Reprogramming Macrophages to M1 Phenotype in Photodynamic Immunotherapy,” was published by Angewandte Chemie International Edition.
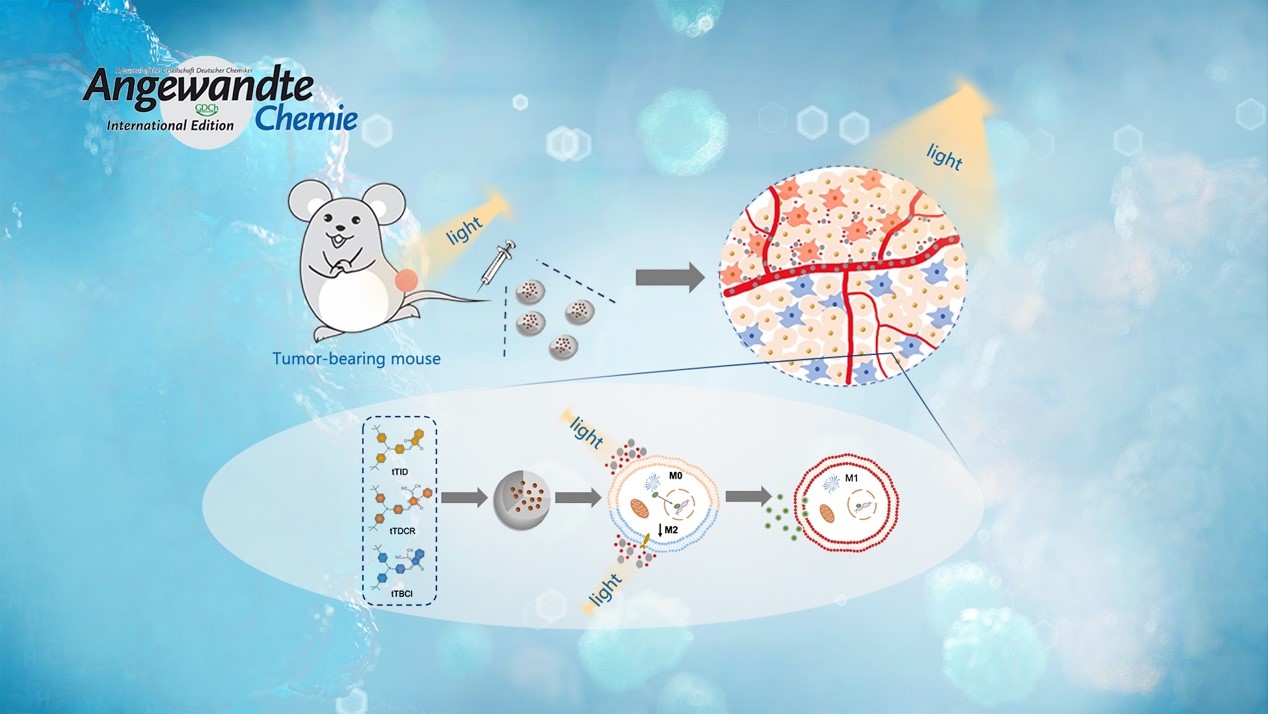
Reprogramming tumor‐associated macrophage cells by photodynamic therapy (PDT) is a promising approach to overcoming the suppression of tumor microenvironment for boosted immunotherapy. It remains unclear how the reactive oxygen species (ROS) generated from type I and II mechanisms relate to the macrophage polarization efficacy. Prof. Li’s team designed and synthesized three photosensitizers with varied ROS‐generating effectiveness.
Surprisingly, they discovered that the extracellular ROS generated from type I mechanisms are mainly responsible for reprogramming the macrophages from a pro‐tumor type (M2) to an anti‐tumor state (M1). In vivo experiments prove that the photosensitizer can produce effective suppression of the tumor growth, while the therapeutic outcome is eliminated with depleted macrophages. Overall, their strategy highlights the design guideline of macrophage‐activated photosensitizers.
Here were the key findings from their analysis:
1. Three donor-acceptor (D-A) structured AIEgen photosensitizers have been synthesized using the compound triphenylamine as the electron donor, and their ROS-generating efficiencies are adjusted by using acceptor units with varied electron deficiencies.
2. Using commercial photosensitizers, such as chlorin 6 (Ce6) and rose bengal (RB), they discover that the extracellular ROS generated from type I mechanism rather than type II mechanism plays a key role in reprogramming the macrophages to M1 phenotype. This is of high importance in in vivo anti-tumor applications, taking into account the lower oxygen dependence of type I PDT mechanism and the hypoxic tumor microenvironment.
3. In vivo results suggest that the tTDCR nanoparticles can lead to a complete removal of a tumor in mice without any relapse, upon a single PDT treatment in the absence of any immune checkpoint inhibitor or immunoadjuvant. This would appear to be attributed to its excellent performance in the activating macrophages.
Dr. Guang Yang, a post-doctoral at SUSTech, is the first author of this paper. Prof. Kai Li, also of SUSTech, is the corresponding author. Jen-Shyang Ni, Yaxi Li, Menglei Zha, and Yao Tu are the co-authors of the paper.
This research was financially supported by the National Natural Science Foundation of China (NSFC), Ministry of Science and Technology of China (MOST), Guangdong Science and Technology Department, and the Shenzhen Science and Technology Program.
Paper Link: https://onlinelibrary.wiley.com/doi/10.1002/anie.202013228
Proofread ByAdrian Cremin, Yingying XIA
Photo By