Assistant Professor Guangfu Luo’s team from the Department of Materials Science and Engineering at the Southern University of Science and Technology (SUSTech) has recently made progress on the stability of lithium-metal batteries. Through classical molecular dynamics simulations and first-principles calculations, the team revealed the atomistic mechanism of using nanoporous materials to reduce the formation of lithium dendrites, its failure mechanism, and the countermeasure. Their study, entitled “Atomistic Mechanism and Long-Term Stability of Using Chlorinated Graphdiyne Film to Reduce Lithium Dendrites in Rechargeable Lithium Metal Batteries,” was published in the renowned journal Nano Letters.
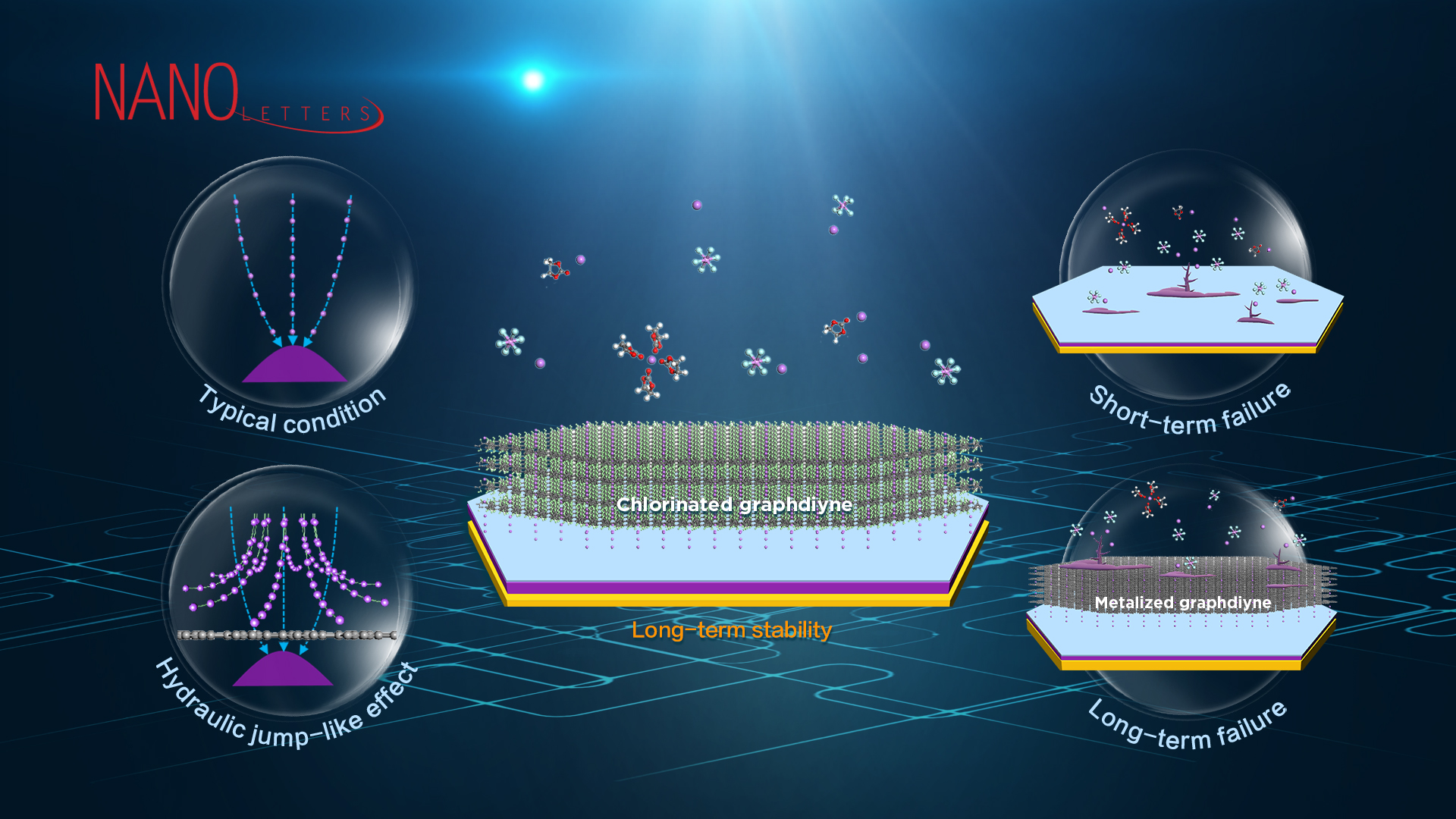
Lithium-metal-anode batteries are the most promising next-generation rechargeable batteries because of their high energy density. They are about ten times that of the commercial graphite-anode batteries, which would significantly prolong the operating time of mobile phones, laptops, unmanned aerial vehicles, mobile robots, electric vehicles, etc. Currently, one overwhelming challenge of commercializing the lithium-metal-anode batteries is the easy formation of massive dendritic crystals, namely the “lithium dendrites.” When the sharp lithium dendrites penetrate the separator during the charging process, a short-circuit catastrophe can be induced. When the lithium dendrites peel off from the roots during the discharge process, “dead lithium” is generated together with a capacity drop. Under typical operating conditions, the state-of-the-art lithium-metal-anode batteries lifespan (80% capacity retention) is about 200 cycles, which is significantly shorter than the 1500-3000 cycles for the commercial graphite-anode batteries. Therefore, inhibiting the growth of lithium dendrites is critical to improving both the safety and lifespan of lithium-metal-anode batteries.
Among the versatile solutions to reduce the growth of lithium dendrites, one promising approach is regulating the lithium ions above anode through porous films to induce a uniform lithium metal growth. However, the atomistic mechanism of this approach and the cause of long-term instability remain largely unknown, which hinders its further improvement. The research team investigated one favorable nanoporous material, graphdiyne, as an example, to reveal the atomistic mechanism of inhibiting the growth of lithium dendrites through molecular dynamics simulations (Figure 1). It was found that graphdiyne induces a hydraulic jump-like effect (like the sink bouncing the flowing faucet water), which can homogenize the lithium ions even under a highly non-uniform electric field and thus induce a uniform lithium metal growth. By contrast, without graphdiyne, the locally stronger electric field on the surface of the lithium anode will attract more lithium ions and induce faster growth of lithium metal, resulting in the continuous growth of lithium dendrites. Since the impact of the hydraulic-like effect increases with the strength of the electric field, a negative feedback is introduced and thus breaks the original causality of lithium dendrites growth.

Figure 1. The geometrical structure of (a) monolayer and (b) bulk graphdiyne. (c) Distribution of a non-uniform electric field used in classical molecular dynamics simulations. Distribution of lithium ions passing through the XY plane (d) with and (e) without graphdiyne film. (f) Statistics of lithium ions passing through a series of annuluses with a fixed area of π (20 Å)2. (g) Trajectories of selected lithium ions before and after touching the graphdiyne film, together with the top view of the initial and final states. All other molecules and ions are hidden for easy visualization.
Through first-principles calculations, the research team further found that the strong reducibility of lithium metal can gradually metalize graphdiyne, allowing lithium metal to nucleate and grow directly on graphdiyne without playing the role of inhibiting lithium dendrites (Figure 2). This discovery reveals the potential failure mechanism in the previous experiments. By examining several modified graphdiynes, the team found that the chlorinated graphdiyne is thermodynamically stable, more resistant to the reduction of lithium, and conducts lithium ions efficiently (Figure 2), which together promises a durable membrane to reduce the growth of lithium dendrites.
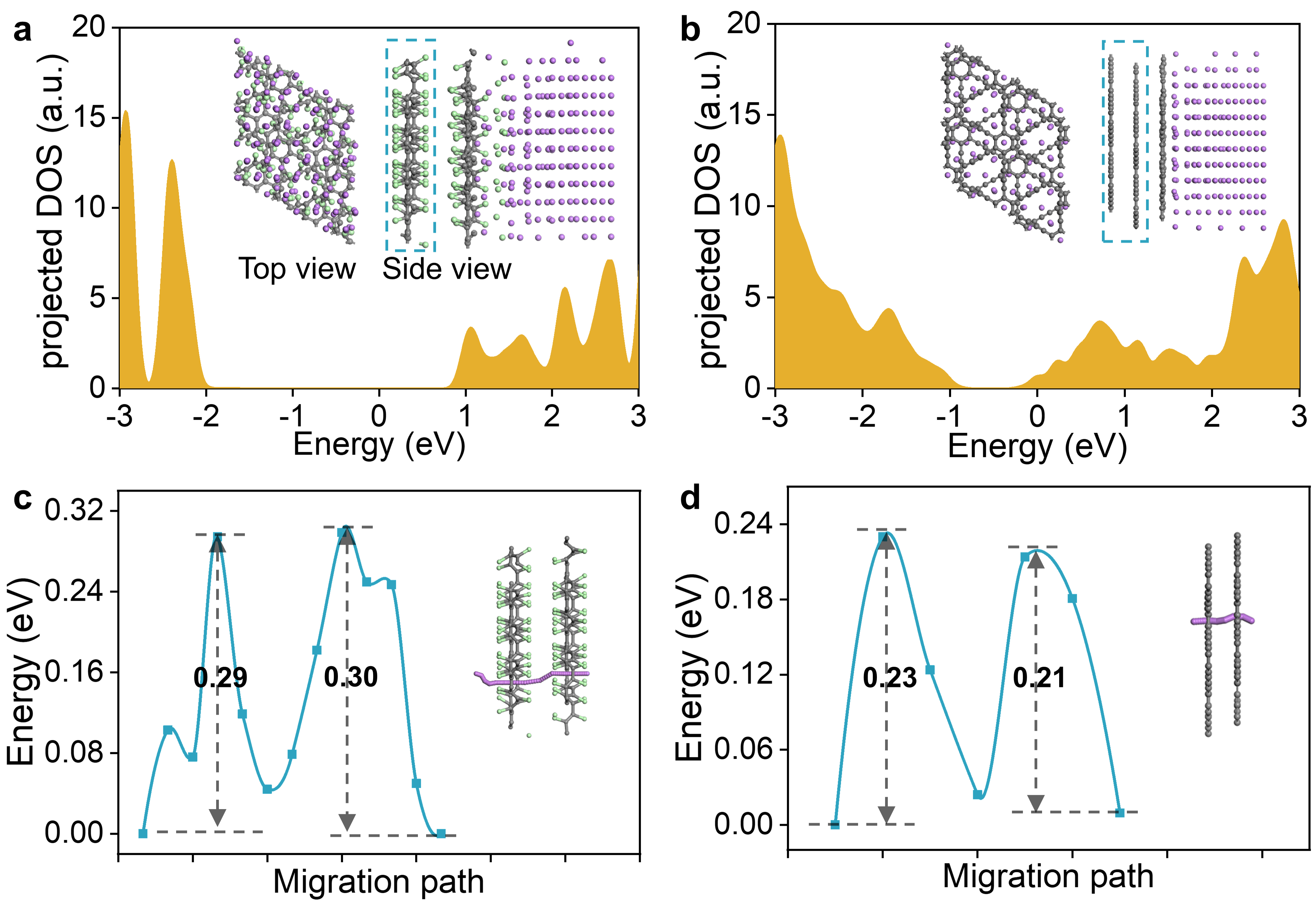
Figure 2. Structure and projected DOS of (a) the fully chlorinated and (b) bare bulk graphdiyne in contact with lithium metal. Energy profiles and hopping paths of lithium-ion through (c) the fully chlorinated and (d) bare bulk graphdiyne.
The atomistic mechanisms and approach revealed in this work would inspire further improvement in the long-term stability of lithium-metal-anode batteries and possibly apply to other rechargeable batteries suffering from the anodic dendrites, such as Na-ion batteries and Zn-air batteries.
Ph.D. student Lina Wang is the first author of this study, and Assistant Professor Guangfu Luo is the corresponding author.
This work was funded by the Introduced Innovative R&D Team of Guangdong and the Guangdong Provincial Key Laboratory of Computational Science and Material Design. All calculations were supported by the Center for Computational Science and Engineering at SUSTech.
Paper link: https://doi.org/10.1021/acs.nanolett.1c02429
Proofread ByAdrian Cremin, Yingying XIA
Photo By