Aprotic LABs (LABs), which employ lithium metal as the anode and oxygen as the cathodic active substances, are a promising energy storage device to significantly boost the endurance mileage of electric transport systems due to its much higher theoretical energy density (3450 Wh kg−1) than that of the current Li-ion batteries (300-500 Wh kg−1). In the recent decade, extensive research efforts have been devoted to this battery technology in terms of the exploration of reaction mechanisms and the improvement of battery performance. Therefore, some significant progress has been achieved. However, the detailed oxygen reduction reaction (ORR) chemistry, especially unambiguous identification of various discharge products and their specific distribution, is still largely unknown or in controversy.
A team led by Associate Professor Meng Gu from the Department of Materials Science and Engineering at the Southern University of Science and Technology (SUSTech) has recently made critical progress in the field of metal-air batteries. Their study, entitled “Revealing the intrinsic atomic structure and novel chemistry of amorphous Li-containing products in Li-O2 batteries using cryogenic electron microscopy,” has been published in the Journal of the American Chemical Society (JACS), a top international academic journal in chemistry.
Crystalline Li2O2 has been well recognized as the primary discharge product at low current density, commonly with a typically toroidal structure. The size of toroidal discharge products usually depends on applied current density (potential), electrolyte composition, and the composition and structure of applied oxygen electrodes. Toroidal products were also reported to contain plate-like crystallite components based on the results from traditional room-temperature transmission electron microscopy (TEM) measurements. Additionally, the formation of large faceted particles is attributed to layer-by-layer growth on existing crystal faces of Li2O2 under a kinetically controlled regime.
Simultaneously, in the in situ X-ray diffraction (XRD) tests, the growth of Li2O2 occurs at a constant rate as reported by a time-dependent Li2O2 diffraction peak area change. In contrast, the decomposition rate of Li2O2 is much slower in the initial stage than in the latter stage, suggesting the existence of amounts of amorphous or parasitic products on the surface of toroidal particles. Besides, synchrotron X-ray transmission microscopy results imply that toroidal particles’ inner core has a dominant Li–O phase, and the external coverage shell contains carbonate species. Yet, the proportion of crystalline and amorphous composition in the toroidal particles, as well as their specific distribution of different phases, are still unavailable up to now. The clarification of this issue is conducive to further understanding the ORR chemistry and inspires researchers to improve the battery performance using more effective measures.
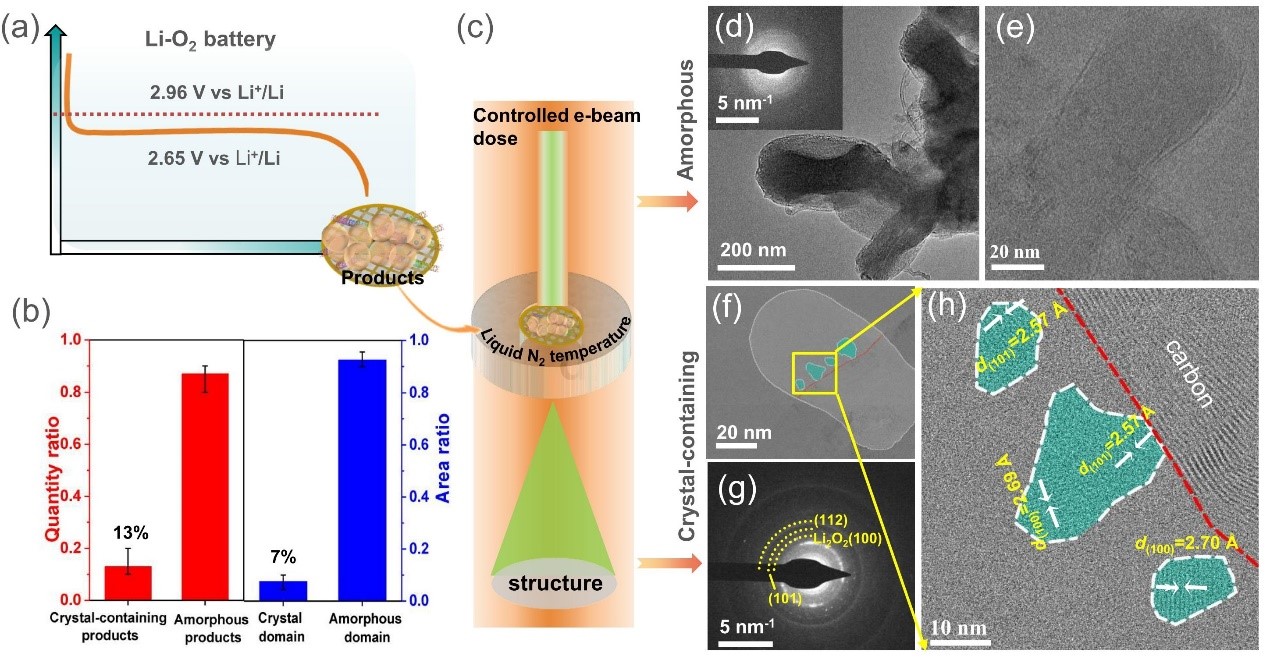
Figure 1. Schematic sample preparation and testing, and high-resolution TEM images under cryogenic conditions
This study demonstrates that electron beam irradiation could easily induce crystallization of amorphous discharge products, which means the need for cryogenic conditions and low beam dose for reliable TEM characterization. Under both liquid-N2 temperature and low electron beam irradiation condition, researchers found that most ORR discharge products are amorphous, and only a few of them contains tiny crystalline Li2O2 domains (Figure 1).
The crystalline domains account for approximately 7% of the total area of the investigated crystal-containing particles (Figure 1b). Therefore, the crystalline portion of all discharge products is only approximately ~0.9%. Furthermore, the amorphous components are identified to be dominated with LiO2 and simultaneously contain amorphous Li2O2 and C-O species by electron energy loss spectroscopy (EELS) (Figure 2). In addition, elements mapping indicates the uniform distribution of lithium, carbon, and oxygen, suggesting the uniform dispersion of byproducts in the toroidal particle.
Interestingly, after a full recharge, most of these toroidal particles could be reversibly decomposed, which presents a superficially high reversibility of LABs. This demonstrates the existence of byproducts as one of the critical reasons leading to the high overpotential during recharge.

Figure 2. Cryo-TEM/EELS and FTIR analysis of the discharge products
This study proves that the determination of sensitive discharge products by Cryo-EELS performs a critical step in the recognition of metal-air batteries. It ensures the accuracy of the reaction mechanism study and the development of new-type and highly efficient metal-air batteries.
Prof. Joseph S. Francisco at the University of Pennsylvania and Dr. Bing Han at SUSTech are the co-corresponding authors of this paper.
This work was supported by the National Natural Science Foundation of China (NSFC), Guangdong Innovative and Entrepreneurial Research Team Program, Shenzhen Science and Technology Program, Guangdong-Hong Kong-Macao Joint Laboratory for Photonic-Thermal-Electrical Energy Materials and Devices. The researchers would also like to acknowledge the support of SUSTech in using the Cryo-Electron Microscopy Center and the SUSTech Core Research Facilities throughout this study.
Paper link: https://pubs.acs.org/doi/full/10.1021/jacs.1c10146
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo By