Silicon-stereogenic arylated silanes and silanols have attracted increasing attention in recent years owing to their unique chemical, physical, biological, and stereoelectronic properties, which have granted them potential applications in the fields of synthetic chemistry, medicinal chemistry, and materials science. However, the catalytic asymmetric synthesis of enantioenriched arylated silanes and silanols is still far from established and full of challenges.
The development of new chemical transformations based on asymmetric catalysis has been the focus of significant research across the world. Recent progress by researchers at the Southern University of Science and Technology (SUSTech) has made new developments in catalytic enantioselective construction of functionalized acyclic silicon-stereogenic arylated silanes and silanols.
In recent years, Associate Professor Chuan He from the Department of Chemistry at SUSTech has led his research group to make significant progress in the catalytic asymmetric dehydrogenative coupling reaction for the construction of silicon-stereogenic silanes (Si-CADC) with high efficiency. Their recent research outcomes regarding the enantioselective synthesis of acyclic silicon-stereogenic arylated silanes and silanols were published in the high-impact academic journal Angewandte Chemie International Edition (Angew. Chem. Int. Ed.).
Direct (hetero)arylation of dihydrosilanes for the synthesis of silicon-stereogenic (hetero)aryl silanes is of great interest and importance due to their utility as fundamental building blocks of functional materials and polymers. Despite numbers of intermolecular C–H silylation of (hetero)arenes with hydrosilanes having been reported, the synthesis of acyclic(hetero) arylsilanes bearing silicon-stereogenic center in one enantiomeric form remains unknown to date.
In the first paper published in Angew. Chem. Int. Ed., entitled “Enantioselective Intermolecular C–H Silylation of Heteroarenes for the Synthesis of Acyclic Si-Stereogenic Silanes”, Prof. He’s team reports the first enantioselective intermolecular C–H silyation of heteroarenes for the synthesis of acyclic silicon-stereogenic heteroarylsilanes (Figure 1).
This process undergoes a rhodium-catalyzed direct intermolecular dehydrogenative Si–H/C–H cross-coupling, giving access to a variety of acyclic heteroarylated silicon-stereogenic monohydrosilanes, including bis-Si-stereogenic silanes, in decent yields with excellent chemo-, regio-, and stereo-control, which significantly enlarge the chemical space of the optically active silicon-stereogenic monohydrosilanes.
Dr. Shuyou Chen and Ph.D. student Jiefeng Zhu, both of the Department of Chemistry at SUSTech, are the co-first authors of this paper. Assoc. Prof. Chuan He is the corresponding author, while SUSTech is the only corresponding unit.
This study was supported by the National Natural Science Foundation of China (NSFC), Guangdong Provincial Key Laboratory of Catalysis, and the Stable Support Plan Program of Shenzhen Natural Science Fund.
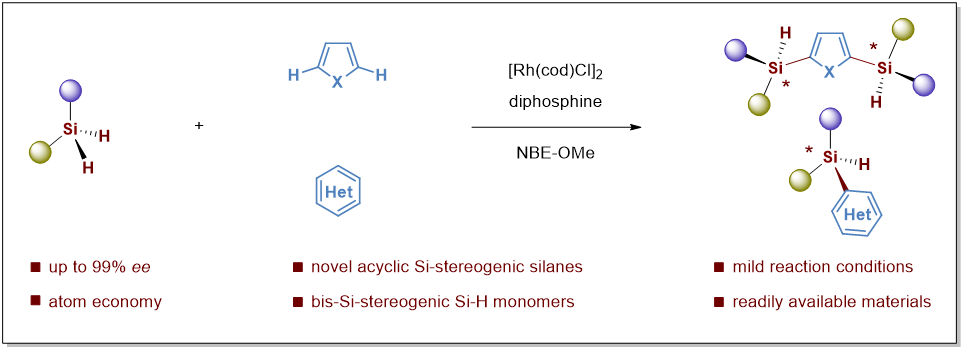
Figure 1. Enantioselective Intermolecular C–H/Si–H Coupling toward Acyclic Si-Stereogenic Silanes
Silanols are silicon analogues of alcohols featuring Si−OH bonds, which are of paramount importance as versatile building blocks to produce silicone polymeric materials. In modern synthetic chemistry, silanols are also widely served as valuable coupling partners, organocatalysts, directing groups, and isosteres of bioactive molecules. Despite growing progress in the construction of chiral organosilicon compounds, the catalytic asymmetric synthesis of silicon-stereogenic silanols is still unknown to date.
The second paper published in Angew. Chem. Int. Ed., entitled, “Synthesis of Si-Stereogenic Silanols by Catalytic Asymmetric Hydrolytic Oxidation”, reports the first enantioselective construction of silicon-stereogenic silanols via catalytic asymmetric hydrolytic oxidation of dihydrosilanes (Figure 2).
This practical protocol features ambient reaction conditions, high atom economy, good functional group compatibility, and a cleaner manner with H2 as the only byproduct, producing a wide range of valuable chiral silanols and bis-silanols in decent yields with excellent chemo- and stereoselectivity.
Dr. Wei Yuan and master’s student Xujiang Zhu, both of the Department of Chemistry at SUSTech, are the first authors of this paper. Assoc. Prof. Chuan He is the sole corresponding author, with SUSTech as the sole communication unit.
This study was supported by the NSFC, Guangdong Provincial Key Laboratory of Catalysis, Shenzhen Science and Technology Innovation Commission, and the Stable Support Plan Program of Shenzhen Natural Science Fund.
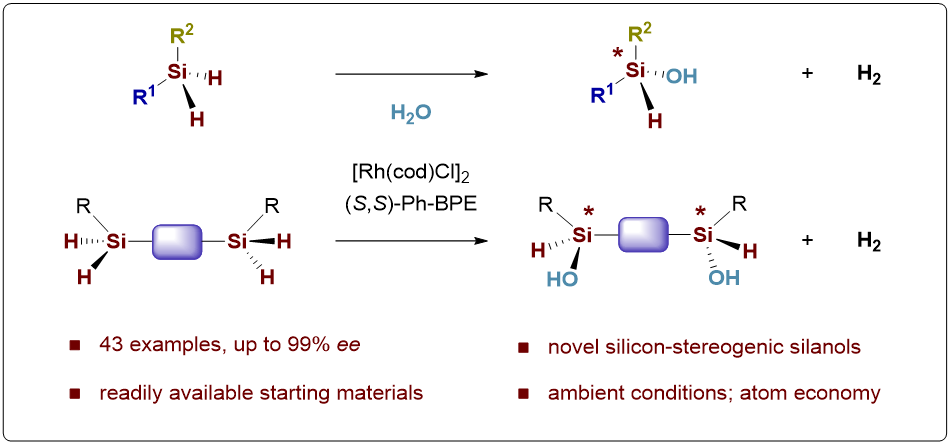
Figure 2. Synthesis of Si-Stereogenic Silanols by Catalytic Asymmetric Hydrolytic Oxidation
Paper links (In order of appearance above):
Angew. Chem. Int. Ed.: https://onlinelibrary.wiley.com/doi/10.1002/anie.202117820
Angew. Chem. Int. Ed.: https://onlinelibrary.wiley.com/doi/10.1002/anie.202204912
Related links:
Prof. Chuan He’s webpage: http://faculty.sustech.edu.cn/hec/
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo By