Inspired by the discovery of an S=N bond in the collagen IV network and its essential role in stabilizing basement membranes, sulfilimines have drawn much attention in the fields of chemistry and biology. Derivatization of peptides and proteins with this S(IV)-derived motif could endow them with novel features. However, its further uptake is hindered by the lack of mild, efficient, and environmentally benign protocols by which sulfilimines can be constructed under biomolecule-compatible conditions.
Assistant Professor Tiezheng Jia’s group from the Department of Chemistry at the Southern University of Science and Technology (SUSTech) and Professor Marisa Kozlowski’s group from the University of Pennsylvania (UPenn) have recently reported a terminal oxidant-free copper-catalyzed dehydrogenative Chan-Lam coupling of free diaryl sulfilimines. The mild reaction conditions and biomolecule-compatible nature allows the employment of this protocol in the functionalization of complex peptides, and, more importantly, as an effective protein bioconjugation method.
Their research results, entitled “Biomolecule-Compatible Dehydrogenative Chan–Lam Coupling of Free Sulfilimines,” have been published in the Journal of the American Chemical Society, a high-impact academic journal.

The suitability of the newly devised copper-catalyzed Chan-Lam coupling for use in sulfilimine-modified peptides was investigated. It displayed excellent chemoselectivity of this protocol, favoring C-N bond coupling of sulfilimine N-H bonds over C-O, C-S, or C-N bond formation of hydroxyls O-H bonds, thiol S-H bonds, amide N-H bonds, or indole N-H bonds. Remarkably, the chemistry was well accommodated with many complex active peptides, including two endogenous opioid receptor agonists, endomorphin-1 and met-enkephalin, and an exogenous opioid receptor agonist, deltorphin I, highlighting the breadth and expediency of this procedure.
Encouraged by the broad tolerance to different peptides, the copper-catalyzed Chan-Lam coupling of free sulfilimines was developed as a selective protein conjugation method. Halotag 7, the 33 kDa monomeric protein, was selected as a model protein substrate for this study, which resulted in the formation of cross-coupling product 7 with 43% yield, confirming the feasibility of the coupling protocol. Moreover, the stability of the S=N bond in protein 7 under various physiologically relevant conditions was tested by Western blots.
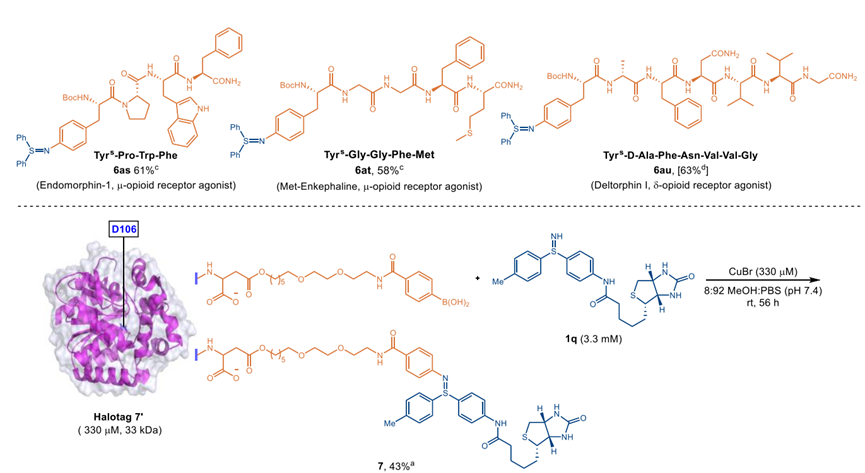
Figure 1. Peptide modification and protein bioconjugation
Based on the combined experimental results and computational studies by Prof. Jia’s and Prof. Kozlowski’s groups, a terminal oxidant-free Chan-Lam coupling mechanism was proposed.
Tingting Meng, a Ph.D. candidate supported by the joint doctoral program between SUSTech and the Harbin Institute of Technology, and Lucille Wells, a Ph.D. student at UPenn, are the co-first authors of the paper. Asst. Prof. Tiezheng Jia from SUSTech and Prof. Marisa Kozlowski from UPenn are the co-correspondent authors.
The authors also acknowledge contributions to this research from undergraduate student Tianxin Wang, Ph.D. student Jinyu Wang, Dr. Shishuo Zhang, and Prof. Jie Wang, all of SUSTech.
This study was supported by the Shenzhen Nobel Prize Scientists Laboratory Project, the Shenzhen Science and Technology Innovation Commission, the Guangdong Provincial Key Laboratory of Catalysis, and the Open Funding Project of the National Key Laboratory of Organic Chemistry Nankai University.
Paper link: https://pubs.acs.org/doi/10.1021/jacs.2c04627
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo By