At present, the treatment methods for plastic waste are mainly landfill, energy recovery, recycling, and reuse. A landfill is to bury plastic waste deeply and waits for natural degradation. This is a slow process, which will cause serious soil pollution. Energy recovery mainly obtains energy through combustion, but treating waste gas generated by combustion requires high technology and cost.
Recycling refers to the degradation of plastics by physical, chemical, or biological methods to obtain oligomers or monomers, which are then used as raw materials for synthetic plastics. Since the covalent crosslinking of the broken ring cannot be restored, the mechanical properties of the recycled materials are always significantly lower than those of the original materials. Reuse refers to the renovation of plastic products by replacing or treating the surface, and the plastic that can be reused accounts for a small proportion.
Ideally, waste plastics should be recycled and transformed into products with higher added value (economic value, energy and environmental value, and functional value), that is, upcycling. Vitrimer, which has both recyclability and complete solvent resistance, may bring a new breakthrough to the upcycling of plastics.

Associate Professor Qi Ge’s research team from the Multifunctional Additive Manufacturing and 4D Printing Laboratory at the Southern University of Science and Technology (SUSTech) has recently published their findings where they developed a UV curable recycling (UVR) solution system that is simple and universal for upcycling vitrimer waste.
This study, entitled “Solvent-Free Upcycling Vitrimers through Digital Light Processing-Based 3D Printing and Bond Exchange Reaction,” was published in Advanced Functional Materials. It was also selected as the back cover of the journal.
Since the concept of vitrimer was put forward in 2011, many vitrimer materials have been developed, and the recyclability of materials has been discussed in these studies. However, it is limited to recycling vitrimer by remodeling, welding, repair, hot pressing, or solvent degradation (Fig. 1a). As an advanced processing technology, 3D printing can realize high-precision and high-complexity manufacturing. Using 3D printing to recycle vitrimer may be a feasible solution to the problem of high-precision and complex molding in plastic recycling manufacturing.

Figure 1. Recycling vitrimers through DLP-based 3D printing and bond exchange reaction
Prof. Ge’s team proposed a method to upcycle vitrimer through digital light processing (DLP) 3D printing and bond exchange reaction (BER). The crushed and ground vitrimer powder is mixed into a UV-curable recycling (UVR) solution to make a low viscosity recycling precursor suitable for DLP 3D printing (Fig. 1b). After adding dispersant, the powder is dispersed evenly and does not produce clusters and precipitation.
Using DLP, the complex and high-precision structures were printed (Fig. 1c), and then complete the bond exchange reaction between vitrimer powder and UVR through thermal curing to form interface fusion (Fig. 1d). Through 3D printing and bond exchange reaction, the waste vitrimer is manufactured into a high-precision complex product. Finally, the upcycling of vitrimer is realized.
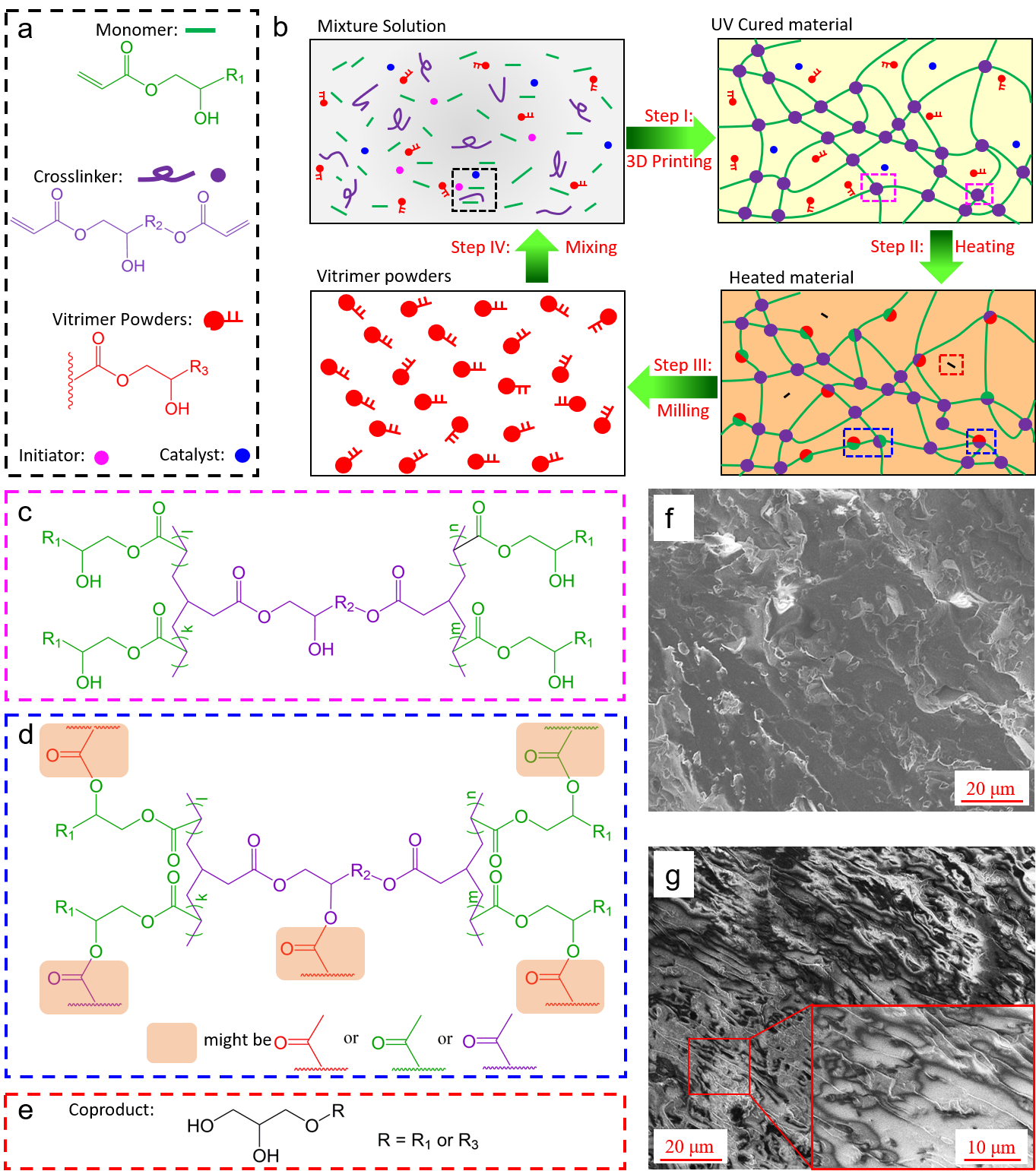
Figure 2. Materials and upcycling mechanism
Figure 2 depicts the chemical details of the vitrimer upcycling. The UVR solution is designed through a two-step polymerization strategy. Figure 2a shows the possible chemicals for preparing the UVR solution. Monomers have mono-functional acrylates for constructing linear chains during 3D printing. The crosslinker agent has bifunctional acrylate, which is used to crosslink the linear chain during 3D printing to form a polymer network.
The vitrimer powder is enveloped by the polymer network (Fig. 2a, 2b step I, 2c, 2f). More importantly, both monomers and crosslinkers have ester and hydroxyl functional groups. They participate in BER based on Transesterification during heating treatment, which covalently integrates the vitrimer molecule into the 3D printing polymer network and further enhances the mechanical properties of the printing structure (Fig. 2b step II, 2d, 2g).
Mechanical tests show that BER caused by heat treatment can improve or regulate the mechanical properties of materials. Suppose the upgraded vitrimer structure needs to be recycled again. In that case, it is possible to grind the structure into powder, mix the powder into UVR solution, and prepare the precursor solution of UVR vitrimer powder mixture again for subsequent recycling (Fig. 2b).
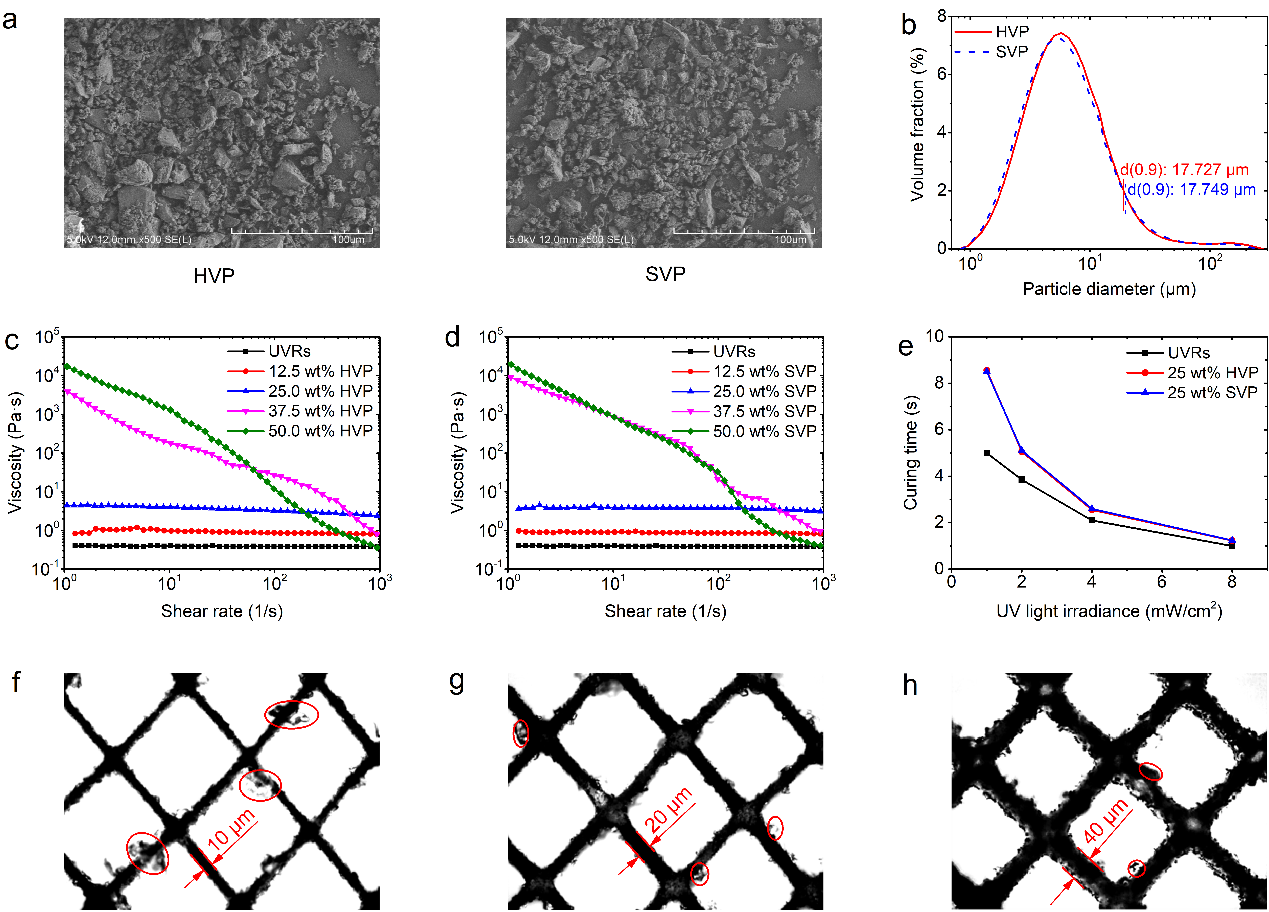
Figure 3. Characterization of vitrimer powders and UVR–vitrimer mixture solution
Preparing a mixed precursor solution consisting of UVR solution and vitrimer powder is a key step in the upcycling vitrimer through DLP-based 3D printing and BER. Therefore, it is crucial to study the properties of vitrimer powder and the precursor solution.
In Figures 3a and 3b, the particle size of most powders is less than 18 µm. When the powder content does not exceed 25 wt.%, the mixed solution is a Newtonian fluid with a viscosity of less than 4 Pa·s (Fig. 3c). As shown in Figure 3e, the pure UVR solution shows excellent photoactivity. The UVR solution containing 25 wt.% powder has relatively weak photoactivity, so it can still be cured quickly under weak ultraviolet light. The above shows that UVR solution with 25 wt.% powder is suitable for DLP-based 3D printing.
The powder particles in the mixed precursor solution affect the resolution of the printing structure. The exposed 10 microns wide grid has obvious particle inlay, and the grid is not smooth and continuous (Fig. 3f). When the mesh width increases to a scale equivalent to the size of the powder (20 µm and 40 µm), the smoothness and continuity of the mesh rod will be greatly improved (Fig. 3g and 3h).
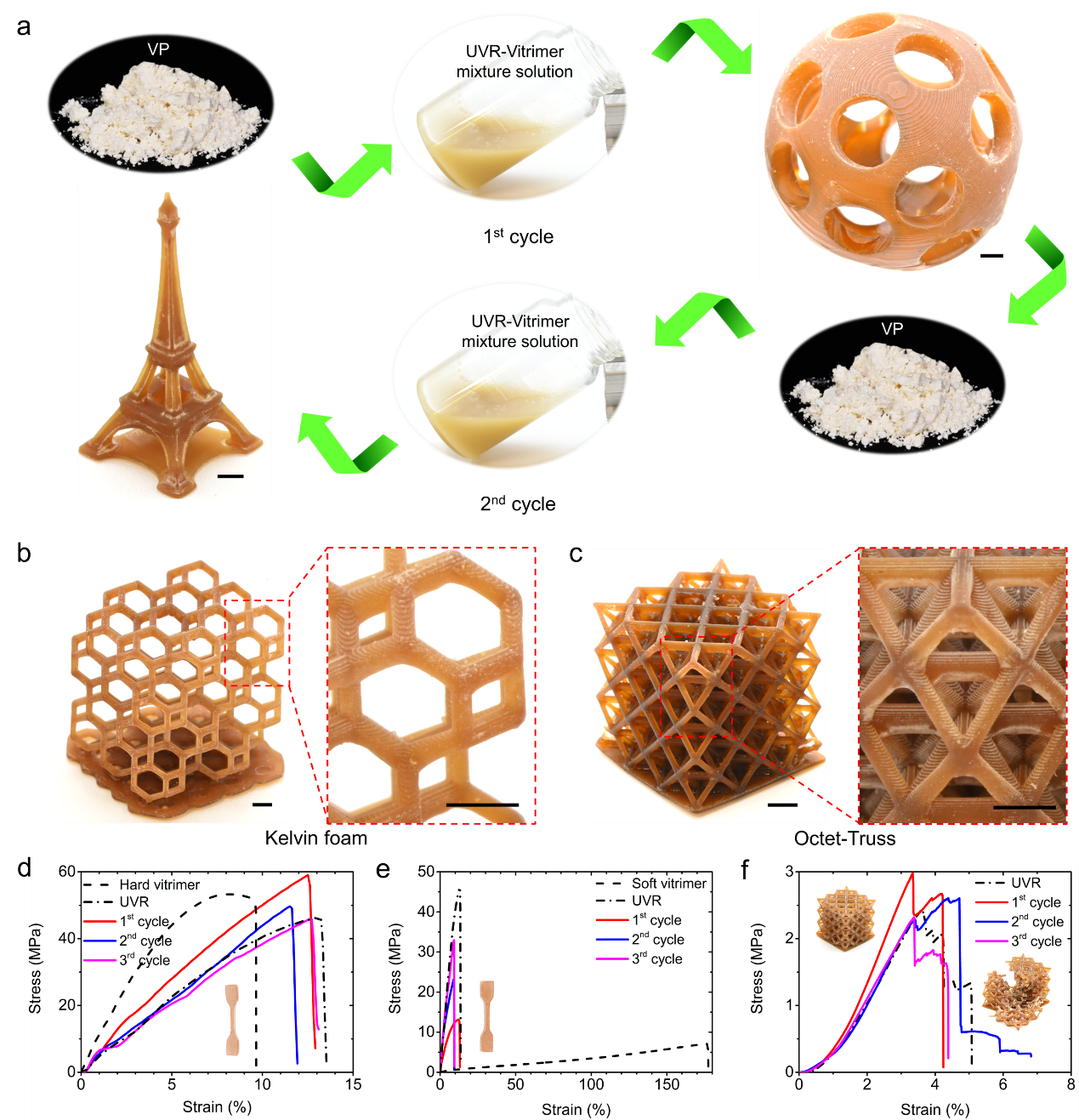
Figure 4. Multiple upcycles of vitrimers with high-precision and high complexity manufactured through DLP 3D printing and bond exchange reaction
The proposed method can be used to upcycle vitrimer many times (Fig. 4a). The UVR vitrimer mixed solution can be used to print high-resolution and highly complex lattice structures (Fig. 4b and 4c). The mechanical properties of the repeatedly recovered UVR hard vitrimer samples are similar to those of the samples made of pure UVR. After multiple cycles, the modulus of the recovered samples reaches equilibrium near the UVR’s modulus (Fig. 4d).
Due to the significant difference in mechanical properties between soft vitrimer and UVR materials, the mechanical properties of the materials gradually approach the properties of UVR with the increase in recycling times (Fig. 4e). By compressing the octet truss structure, the changes in mechanical properties of the structure of 3D printing with multiple upcycles were further studied (Fig. 4f). The above tests show that this method can form a complex 3D structure by upcycling the vitrimer without sacrificing mechanical properties.
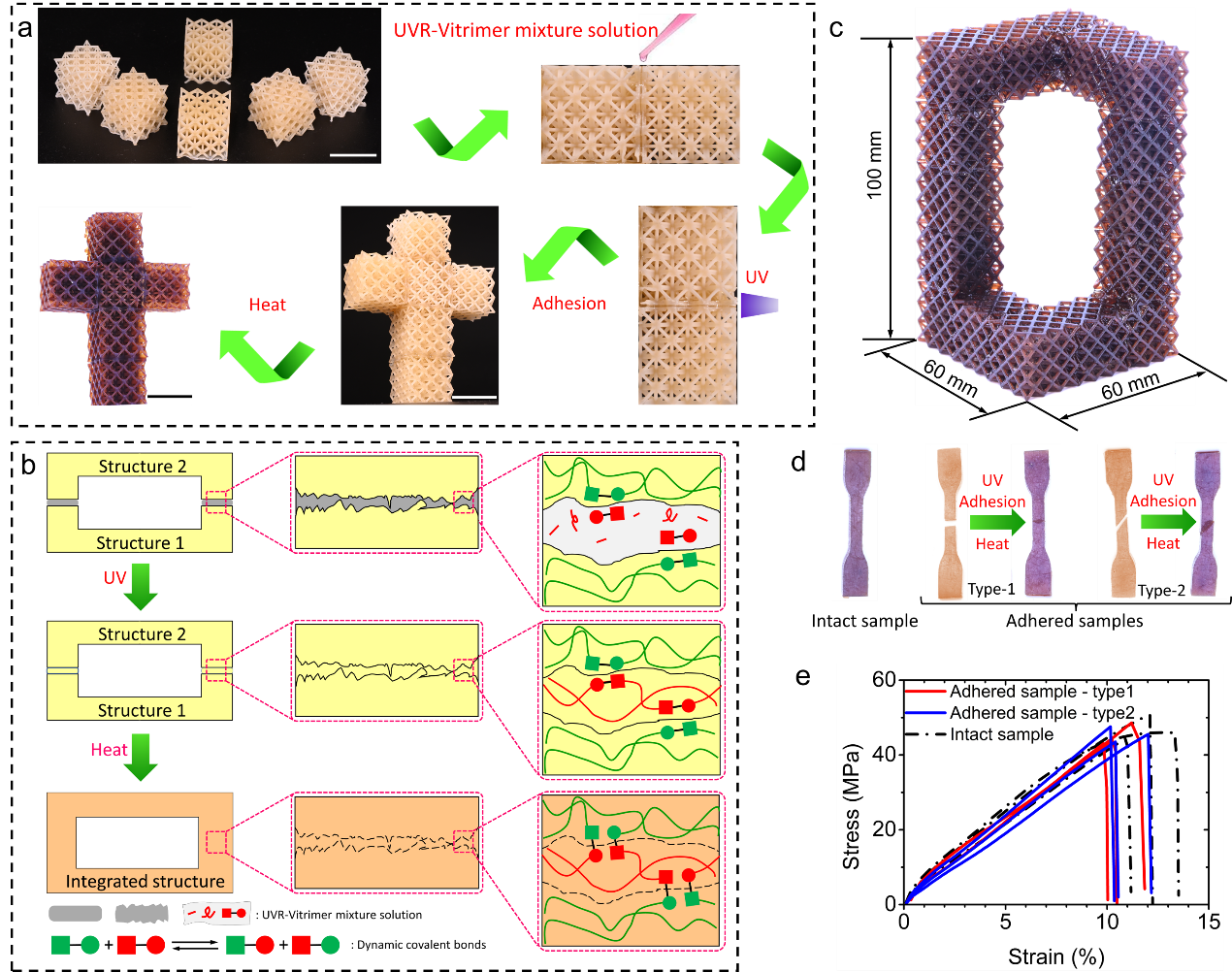
Figure 5. Unsupported large-scale, high-precision 3D components are realized through UV curing adhering and thermally induced bond exchange reaction
The prepared UVR-vitrimer mixture solution can print the 3D structure directly and glue the separately printed parts together to build a large-scale, high-precision, unsupported, and highly complex 3D model (Fig. 5a and 5c). The detailed bonding process and mechanism are also displayed (Fig. 5b). The influence of the bonding interface on the mechanical properties of the material is judged by comparing the complete dumbbell sample and the adhesive sample (Fig. 5d).
The results showed that the mechanical properties of the adhesive samples were almost the same as those of the intact samples. The adhesive samples with different interface types show nearly the same stress-strain behavior. More importantly, the fracture interface of the adhesive sample does not follow the adhesive interface, which further indicates that this adhesion method can give the adhesive sample a firm interface.
The upcycling method proposed in this study is based on the vitrimer of transesterification reaction, which can also be further extended to other dynamic covalent chemistry. This method brings a new breakthrough to the upcycling of plastics and provides a practical solution to the environmental challenges related to plastic pollution.
Honggeng Li, a postdoctoral fellow at SUSTech, and Biao Zhang, Assoc. Prof. at Northwestern Polytechnical University (NPU) and visiting scholar at SUSTech, are the co-first authors of this paper. Assoc. Prof. Qi Ge is the only corresponding author.
The research was supported by the National Natural Science Foundation of China (NSFC), Key Area Research and Development Program of Guangdong Province, Natural Science Basic Research Program of Shaanxi, and the China Postdoctoral Science Foundation.
Paper link: https://onlinelibrary.wiley.com/doi/10.1002/adfm.202111030
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo By