The transition-metal-catalysed cross-coupling reaction between an organo(pseudo)halide and a nucleophile is important in organic synthesis for carbon-carbon (C–C) and carbon-heteroatom (C–E, where E indicates p-block elements other than carbon) bond formations, in both industrial and academic settings (Fig. 1a).
In stark contrast, synthetic methodologies for the corresponding transition-metal-catalysed heteroatom–heteroatom (E–E’) cross-coupling between a heteroatomic (pseudo)halide and a heteroatomic nucleophile has so far remained largely underexplored, and its enantioselective variant is unknown. The challenge mainly rests on the final reductive elimination step (Fig. 1b).
Professor Xin-Yuan Liu from the Department of Chemistry at the Southern University of Science and Technology (SUSTech) has led his research team to make a breakthrough in a strategy for developing enantioselective S–O coupling under Cu catalysis, based on both experimental and theoretical results.
The paper, entitled “Cu-catalysed enantioselective radical heteroatomic S–O cross-coupling,” was published in Nature Chemistry, one of the preeminent academic journals in chemistry.
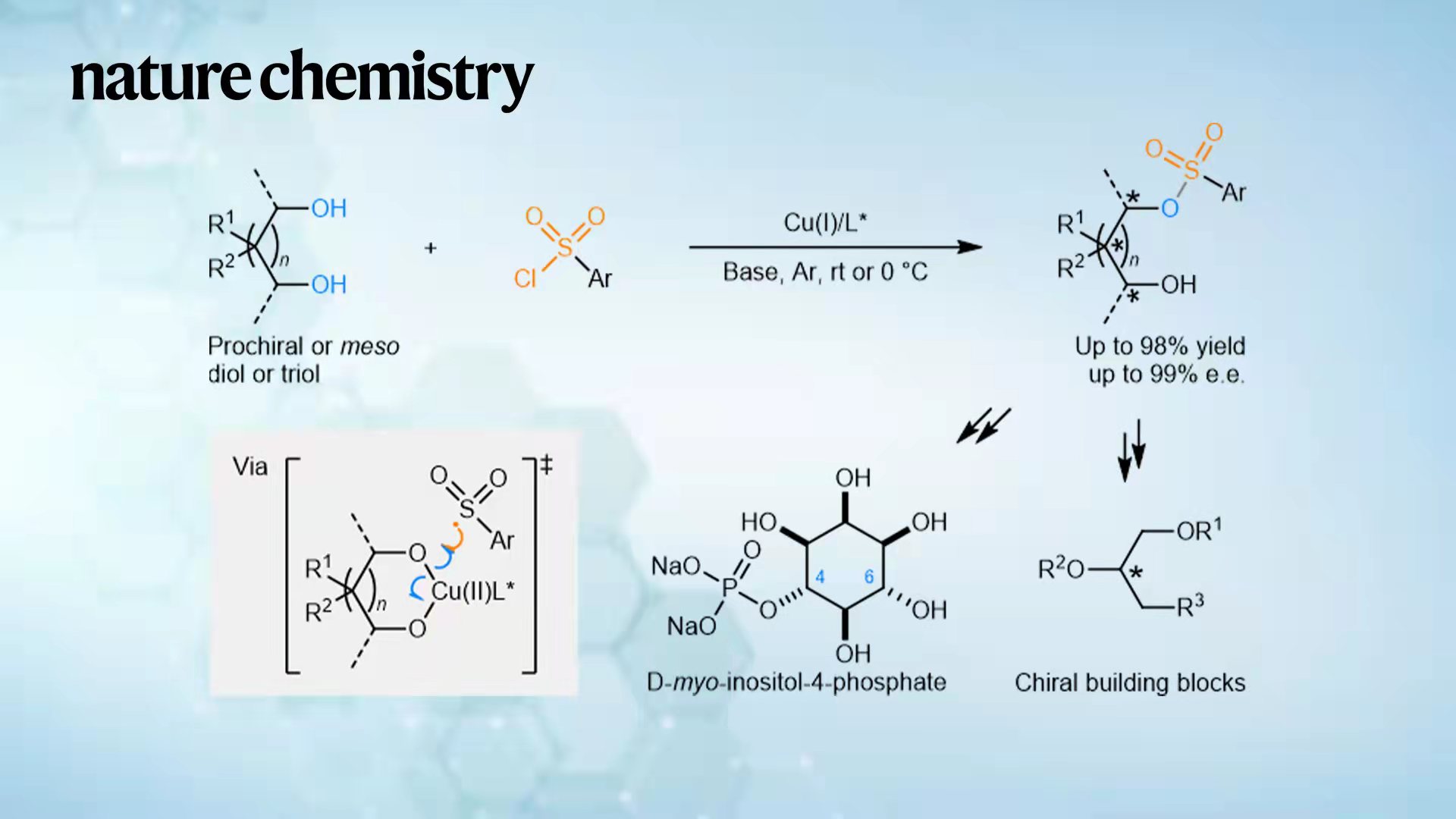
In this study, Prof. Liu’s research team described a copper-catalysed enantioselective S–O coupling reaction that probably proceeds through a rare single-electron E–E′ reductive elimination, on the basis of experimental and theoretical studies (Fig. 1c). The reaction leads to the successful desymmetrization of a variety of prochiral or meso diols or triols.
Accordingly, a panel of highly enantioenriched 1,2-diol, 1,3-diol, 2-amino-1,3-diol, triol, tetraol, pentanol, and hexanol scaffolds with up to six stereocenters were efficiently constructed, in particular, those synthetically challenging acyclic all-carbon quaternary as well as nitrogen- and oxygen-bearing tetrasubstituted carbon stereocenters (Fig. 1d).
These results demonstrate the potential of enantioselective radical heteroatomic cross-coupling as a general chiral heteroatom–heteroatom formation strategy.

Figure 1. Challenges and development of transition-metal-catalysed enantioselective heteroatom–heteroatom cross-coupling. a) An outline of the mechanism and overview of transition-metal-catalysed enantioselective cross-coupling. OA, oxidative addition; LE, ligand exchange; RE, reductive elimination; E, p-block elements other than carbon. b) Challenge for realizing E–E′ cross-coupling via enantioselective RE from transition-metal complexes. c) Copper catalysed enantioselective S–O cross-coupling via single-electron RE. Ar, argon. d) A broad scope of enantioenriched products (>50 examples, with compounds containing up to six stereocenters) formed in this study.
Research Asst. Prof. Yong-Feng Cheng from the Department of Chemistry at SUSTech, Ph.D. students Zhang-Long Yu and Yu Tian at SUSTech, and Ph.D. student Ji-Ren Liu of Zhejiang University, are the first authors of this paper. Prof. Xin-Yuan Liu from the Department of Chemistry at SUSTech, Research Assoc. Prof. Qiang-Shuai Gu at SUSTech, and Prof. Xin Hong of Zhejiang University, are the corresponding authors.
This work was supported by the National Natural Science Foundation of China (NSFC), Guangdong Innovative Program, Shenzhen Special Funds, and SUSTech Special Fund for the Construction of High-Level Universities.
Paper link: https://www.nature.com/articles/ s41557-022-01102-z
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo By