α-Chiral alkyl thiols and other organosulfur moieties with different sulfur oxidation states are important chiral building blocks in organic synthesis and biochemical processes, as well as key structural elements in a variety of biomolecules, pharmaceuticals, and agrochemicals. Consequently, the efficient catalytic enantioselective carbon–sulfur (C(sp3)–S) bond formation constitutes a longstanding objective in modern chemical and biological research.
Chair Professor Xin-Yuan Liu from the Department of Chemistry at the Southern University of Science and Technology (SUSTech) has led his research team to make a breakthrough in a strategy for developing enantioconvergent C(sp3)–S cross-coupling under copper catalysis, based on both experimental and theoretical results.
Their paper, entitled “A general copper-catalysed enantioconvergent C(sp3)–S cross-coupling via biomimetic radical homolytic substitution,” has been published in Nature Chemistry.
Among a range of options, asymmetric organocatalysis and Lewis acid catalysis have been extensively investigated for developing highly enantioselective C–S bond formation with sulfur nucleophiles. In stark contrast, the transition-metal-catalysed enantioselective C(sp3)–S bond construction still represents an underdeveloped domain likely due to the difficult heterolytic metal–sulfur bond cleavage (e.g., heterolytic BDE (copper(II)–S) = ~162 kcal/mol) and notorious catalyst-poisoning capability of sulfur nucleophiles (Fig. 1b). In enzymatic biocatalytic processes (Fig. 1a), the key C(sp3)–S bond formation in some cases proceeded preferably through the radical homolytic substitution of a [4Fe–4S] cluster by a carbon-centered radical, likely owing to the relatively low homolytic BDE of a Fe–S bond (BDE = ~61 kcal/mol).
In this work, Prof. Liu’s research team designed a copper/chiral tridentate anionic ligand catalyst that forges the chiral C(sp3)–S bond via a biomimetic radical homolytic substitution of the LCuII–SR complex by the prochiral alkyl radical (Fig. 1c, 1d). The reaction tolerates a range of racemic secondary/tertiary alkyl halides and different types of highly transformable sulfur nucleophiles with high functional group compatibility (Fig. 1e). More importantly, when allied with follow-up transformations, this strategy provides a highly flexible and practical platform to rapidly access an abundant of related organosulfur compounds (Fig. 1f). These compounds include thiol, thioether, disulfide, sulfoxide, sulphone, sulfoximine, sulfonamide, and sulfonyl fluoride. Most common substitution types of α-stereocenters are present in these compounds, making them useful synthetic building blocks, demonstrating the synthetic utility and adaptability of this methodology.
This work provides a promising blueprint for developing enantioselective carbon–heteroatom bond formation reactions with strongly coordinating heteroatomic nucleophiles via the biomimetic radical homolytic substitution pathway.
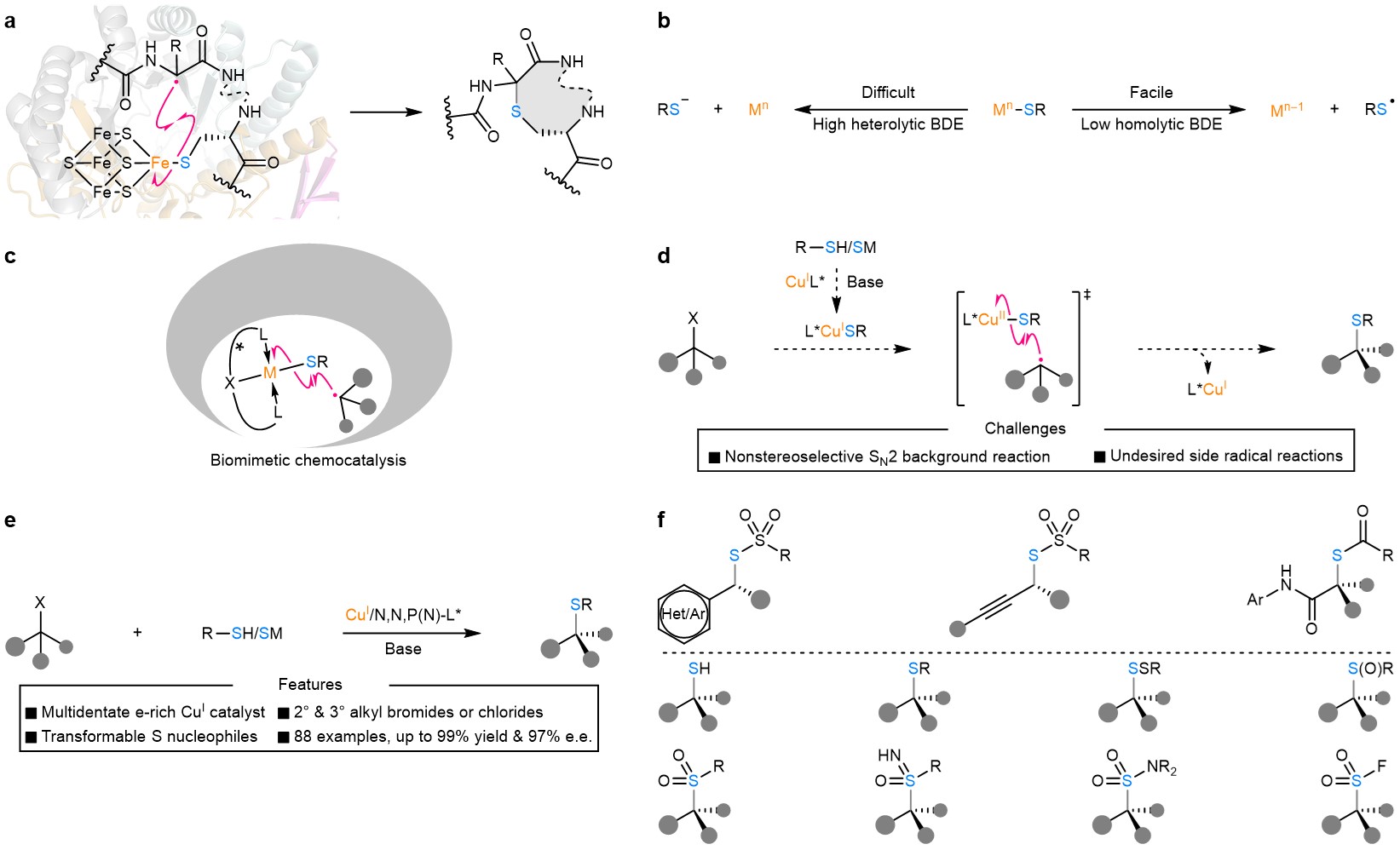
Figure 1. Motivation and design of Cu(I)-catalysed enantioconvergent C(sp3)–S cross-coupling via biomimetic radical homolytic substitution. (a) The rSAM enzyme CteB (PDB code 5WGG) and the enantioselective radical C(sp3)–S bond formation catalysed by it. (b) The heterolytic cleavage of a metal–S bond is usually energetically demanding, while the corresponding homolytic cleavage is commonly facile. (c) A biomimetic transition-metal-catalysed enantioselective radical homolytic substitution-type C(sp3)–S bond formation is highly promising and remains to be explored. (d) A proposed Cu(I)-catalysed enantioconvergent radical C(sp3)–S coupling together with conceivable challenges, such as nonstereoselective SN2 background reaction and undesired side reactions. (e) This work on Cu(I)-catalysed enantioconvergent radical C(sp3)–S coupling of racemic secondary and tertiary alkyl halides with transformable sulfur nucleophiles. (f) This methodology provides expedient access to a panel of α-chiral alkyl organosulfur compounds.
Postdoctoral fellows Yu Tian, Xitao Li, Jiren Liu, and Jian Cheng, and master’s graduate Ang Gao, are the co-first authors of this paper. Chair Professor Xin-Yuan Liu is the corresponding author, and SUSTech is the corresponding unit of the paper.
This work was supported by the National Key R&D Program of China, National Natural Science Foundation of China (NSFC), Guangdong Innovative Program, Shenzhen Special Funds, and the SUSTech Special Fund for the Construction of High-Level Universities.
Paper link: https://doi.org/10.1038/s41557-023-01385-w
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo ByDepartment of Chemistry