The global impact of the Mpox pandemic, caused by the Mpox virus (commonly known as the monkeypox virus, MPXV), has prompted intensified scrutiny. With no officially marketed vaccine or drug for the monkeypox virus in China, there is an urgent need to enhance research on the virus’ infection and pathogenic mechanisms. The D5 protein, identified as a putative helicase-primase found in MPXV, plays a vital role in viral replication and genome uncoating.
Assistant Professor Renhong Yan’s research team from the Department of Biochemistry at the Southern University of Science and Technology (SUSTech) has recently published their latest findings elucidating the mechanism of the regulation of C-terminal helicase activity by the N-terminal initiator enzyme of the D5 protein. The molecular mechanism of ATP hydrolysis and DNA unwinding in poxvirus were clarified, which has made an important contribution to understanding the mechanism of D5 unwinding.
Their work, entitled “Structural insight into the assembly and working mechanism of helicase-primase D5 from Mpox virus”, has been published in Nature Structural & Molecular Biology.
The researchers employed the high-resolution cryo-EM structures of the full-length D5 in various states, which reveal the characteristics of D5 movement along single-stranded DNA (single-stranded DNA, ssDNA) during ATP hydrolysis, shedding light on the molecular mechanism of ATP hydrolysis and DNA unwinding in poxvirus. However, due to the insufficient understanding of the working mechanism of poxvirus replication machines, the development of relevant antiviral drugs has been greatly limited. Thus, this study focuses on the monkeypox virus helicase-initiator enzyme D5, which is a good broad-spectrum antiviral drug target because it is highly conserved among different poxviruses.
In their exploration of D5’s unwinding activity, the team discovered that the full-length D5 lacks persistent DNA unwinding activity. Persistent DNA unwinding activity was only observed after removing the initiator enzyme domain or disrupting the interaction between the initiator enzyme domain and the helicase domain (Figure 1). This indicates that the N-terminal initiator enzyme domain plays a role in regulating the C-terminal helicase activity.
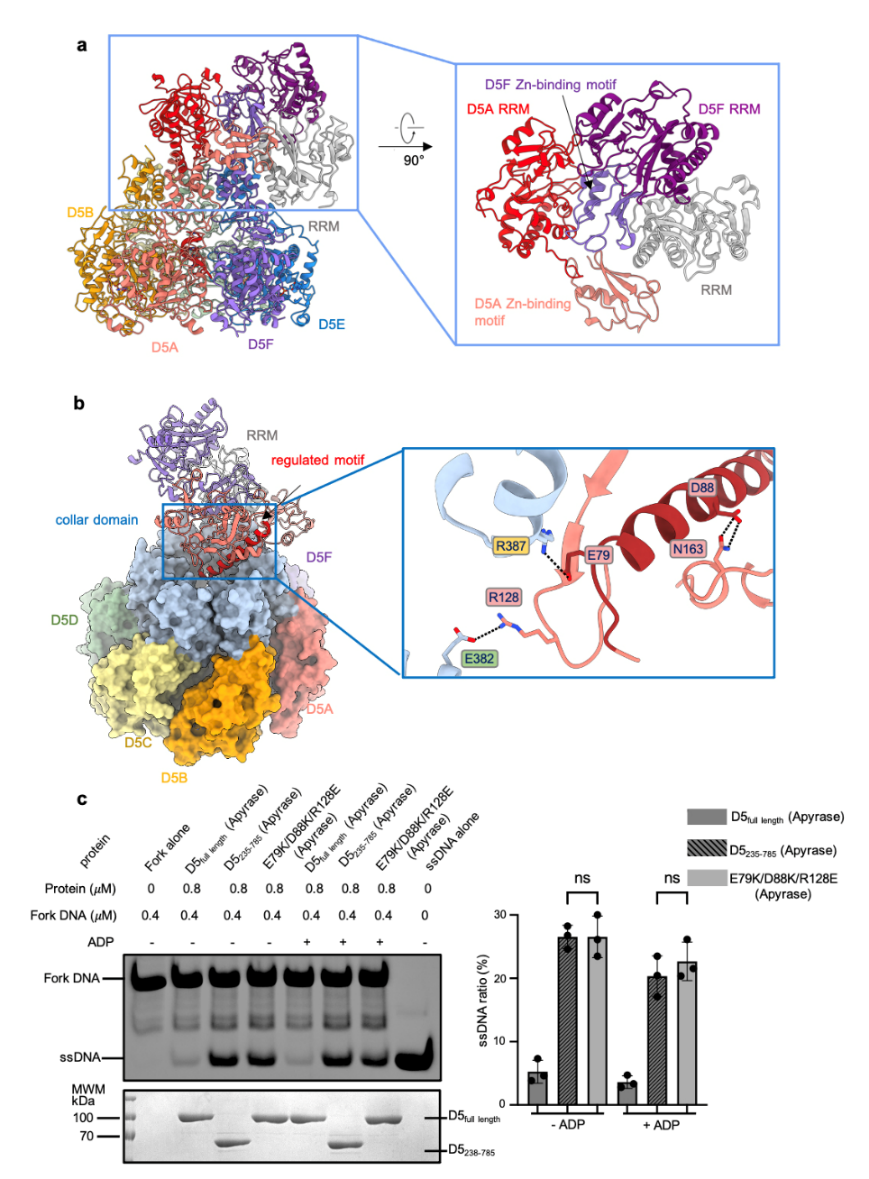
Figure 1. Structural analysis of N-terminal domain of D5
The structure analysis revealed two intermediate states of DNA unwinding in conjunction with ATP, proposing a six-polymeric D5 model that consumes ATP to regulate the interaction between each monomer and adjacent monomer, completing the process of the DNA unwinding model (Figure 2).
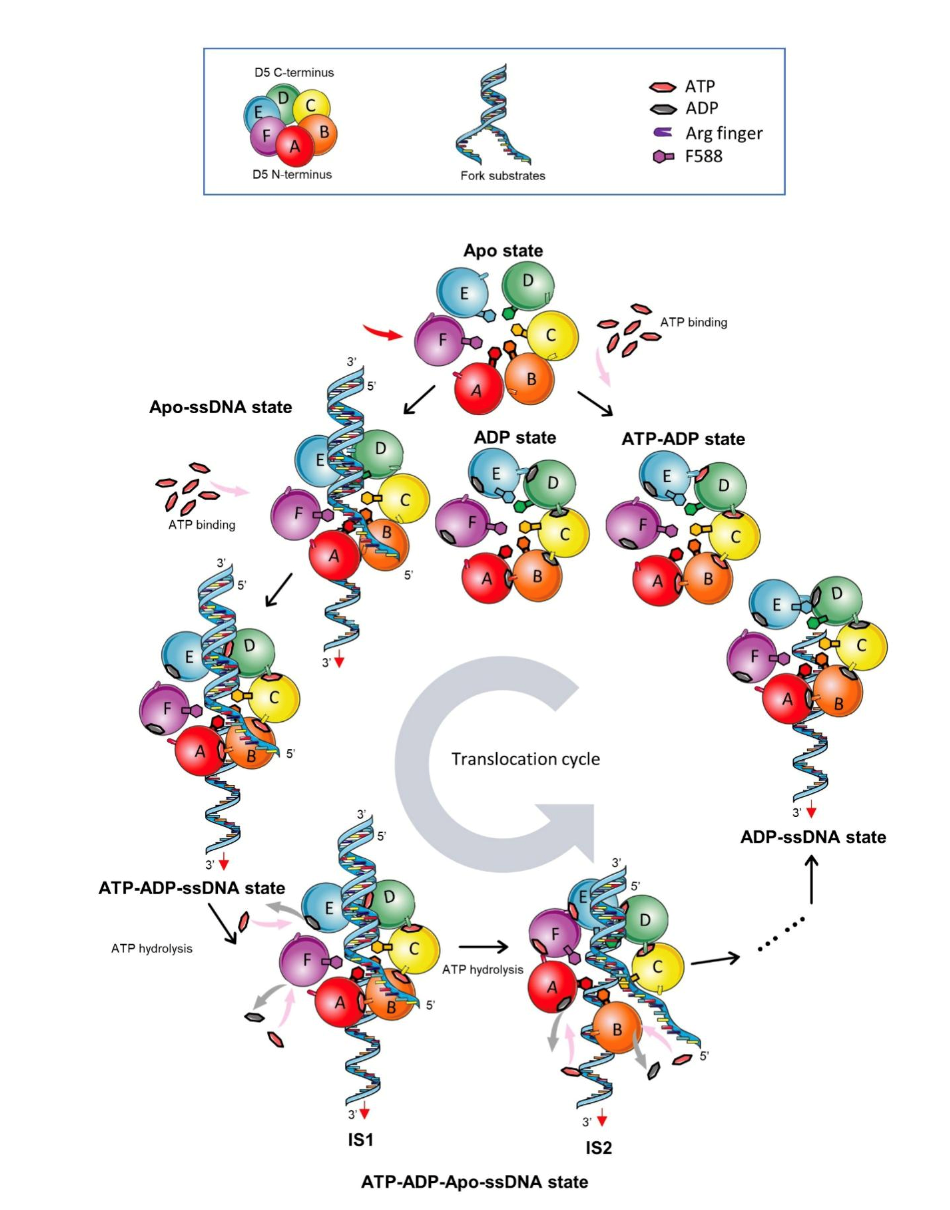
Figure 2. A proposed mechanism of ssDNA translocation from D5
In conclusion, this study found the regulatory mechanism of C-terminal helicase activity by the N-terminal initiation enzyme of the D5 protein, clarifying the molecular mechanism of ATP hydrolysis and DNA unwinding in poxvirus. This research not only contributes significantly to understanding the mechanism of D5 unwinding, but also serves as a crucial foundation for the development of broad-spectrum drugs against pox viruses.
It is worth mentioning that Professor Renhong Yan’s team, in early 2023, also resolved the assembly and working mechanism of the monkeypox virus replicase enzyme, further deepening our understanding of the DNA replication mechanism in the pox virus infection process and providing key insights for subsequent drug development against pox viruses.
Yaning Li and Jing Zhu, Ph.D. students from Tsinghua University, are the co-first authors of this paper. Assistant Professor Renhong Yan and former Research Associate Professor Yingying Guo from SUSTech (now a researcher at the School of Life and Health at Hainan University) are the co-corresponding authors.
The study was supported by the Science, Technology and Innovation Commission of Shenzhen Municipality and the National Natural Science Foundation of China (NSFC). The researchers also acknowledge the Cryo-Electron Microscopy Center of SUSTech for providing technical support in EM data acquisition.
Paper link: https://www.nature.com/articles/s41594-023-01142-0
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo ByDepartment of Biochemistry