During the long-term co-evolution with bacteriophages, bacteria have evolved a series of immune systems to defend against bacteriophage invasion. Simultaneously, bacteriophages have also developed strategies to evade bacterial immune systems. However, despite the progress made in understanding this evolutionary “arms race”, many questions remain unanswered.
Further research in this field is crucial to unraveling the intricate mechanisms underlying bacterial-phage interactions, shedding light on novel strategies employed by both parties and potentially offering insights into the development of innovative antimicrobial therapies.
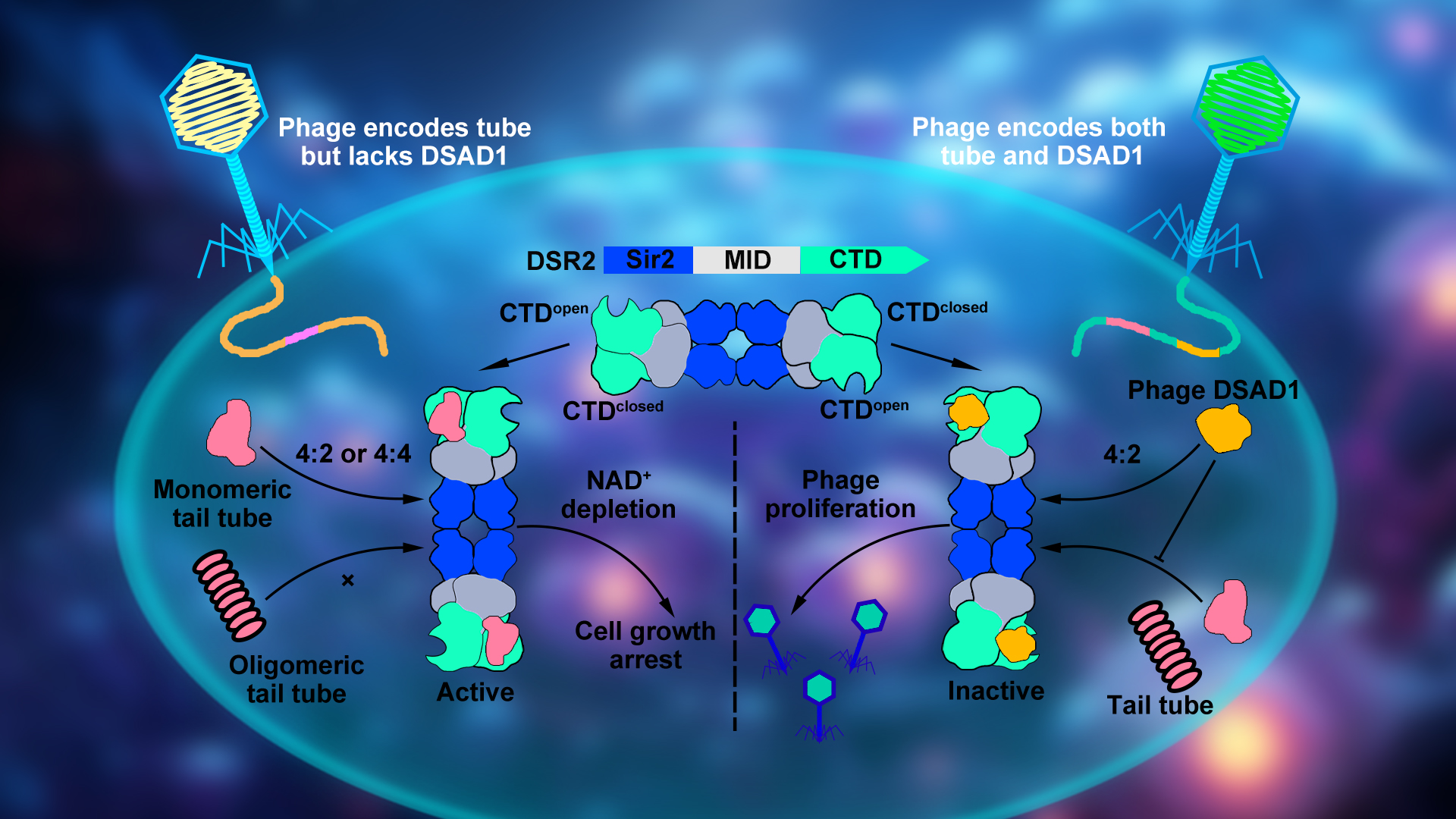
Associate Professor Ning Jia’s research group from the School of Medicine at the Southern University of Science and Technology (SUSTech) has recently published a study revealing that the bacterial Defense-associated sirtuin 2 (DSR2) immune system activates its own NADase domain by recognizing the invading bacteriophage’s tail tube protein. This process depletes its own NAD+ and initiates a suicide death to protect the community. Furthermore, the study sheds light on how “clever” bacteriophages use their DSR anti-defense 1 (DSAD1) protein to suppress the bacterial immune system, thereby enabling them to escape from bacterial immune attacks. This research enhances our understanding of the ongoing “arms race” between bacteria and bacteriophages.
Their work, entitled “Structural basis for phage-mediated activation and repression of bacterial DSR2 anti-phage defense system”, has been published in Nature Communications.
The group focused on elucidating the mechanism of the prokaryotic immune systems against the invading phages and plasmids. Over recent years, they have unveiled the molecular mechanisms of various prokaryotic immune systems, including CRISPR and pAgos systems, in defending against phage and plasmid invasions and have published a series of related papers (Nature Chemical Biology, 2023; Molecular Cell, 2023, 2019a, 2019b, 2019c; Nature Communications, 2022; Science, 2020; Cell Research, 2020, 2022; Nature Reviews Molecular Cell Biology, 2021).
The DSR2 protein has a highly conserved Silent Information Regulator 2 (Sir2) domain. The Sir2 family of proteins is an ancient and widely distributed family of NAD+-dependent enzymes that play crucial roles in various important biological processes. Recent studies have shown that the DSR2 protein from Bacillus subtilis can recognize bacteriophage tail tube proteins, activating its Sir2 domain to deplete intracellular NAD+, leading to growth arrest or death of infected cells. Additionally, to suppress the bacterial DSR2 immune system, bacteriophages encode the DSAD1 protein, which can directly bind and inhibit DSR2. However, the molecular mechanisms by which bacteriophages activate and inhibit the DSR2 immune system remain unknown.
To elucidate the molecular mechanisms by which bacteriophages activate or suppress the DSR2 immune system, the researchers first conducted physiological experiments to confirm that the co-expression of DSR2 and the tube protein leads to bacterial growth inhibition (Figure 1). Biochemical experiments revealed that DSR2 can form a stable multimer. Using Cryo-EM single-particle analysis, they resolved the high-resolution structure of DSR2 and discovered that the DSR2 protein forms a head-to-head tetramer. Beyond the Sir2 domain, DSR2 also possesses a MID domain and a CTD domain, and structural comparisons revealed that within the DSR2 tetramer, the CTD domain exists in “open” and “closed” states.
The group demonstrated through in vitro biochemical experiments that only the monomeric state of the tube protein can activate DSR2’s NADase activity. They obtained the DSR2-tube complex using in vitro co-purification and determined its high-resolution structure. Structural analysis revealed that in the DSR2-tube complex, the monomeric state of the tube protein binds to the CTD domain in the “closed” state (Figure 1).
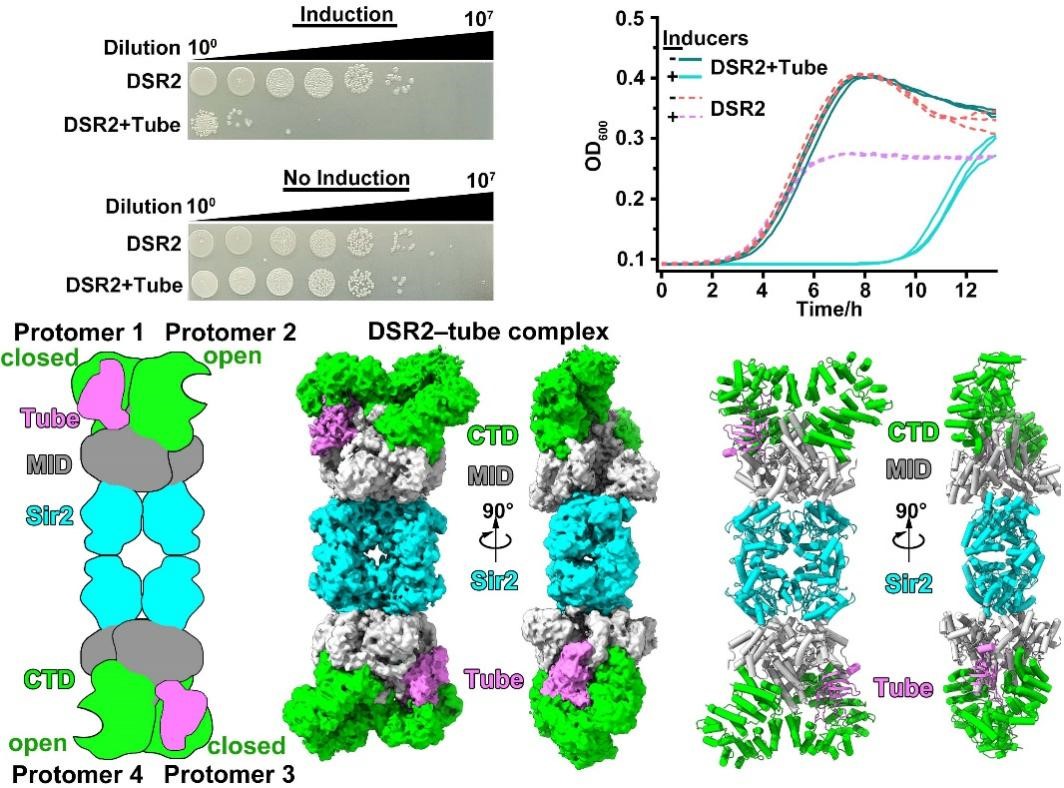
Figure 1. Tube proteins could bind to and activate the NADase activity of DSR2
Additionally, through physiological and biochemical experiments, the researchers discovered that when the bacteriophage-encoded DSAD1 protein and the tube protein are expressed simultaneously, DSR2 cannot be activated by the tube protein, and its NADase activity is inhibited (Figure 2). To explore the molecular mechanism by which DSAD1 protein inhibits DSR2, they further determined the Cryo-EM structure of the DSR2-DSAD1 complex. They found that unlike the tube protein, which prefers to bind to the CTD domain in the “closed” state, the DSAD1 protein tends to bind to the CTD domain in the “open” state. Further analysis revealed that the β1 strand of DSAD1 interacts with the DSR2 protein, preventing the conformational change of DSR2, thus allosterically inhibiting the binding between DSR2 and the tube protein, allowing the bacteriophage to escape the DSR2 immune system (Figure 2).
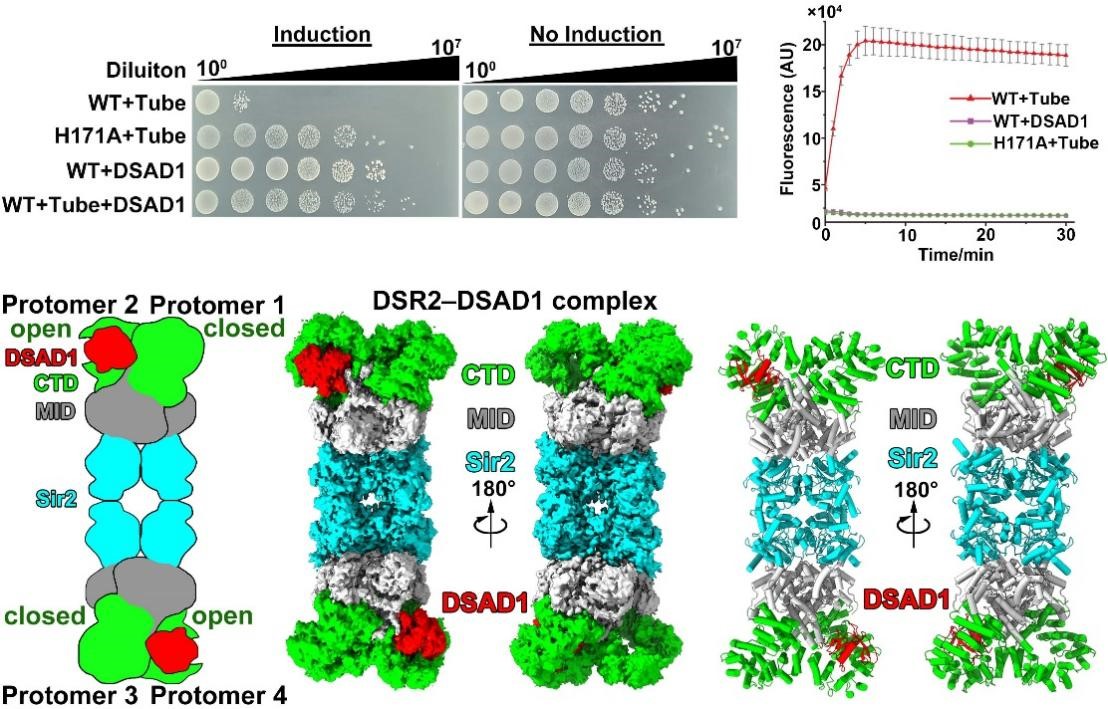
Figure 2. DSAD1 could bind to DSR2 and prevent its binding to tube proteins
In summary, the researchers proposed a dynamic model of the DSR2 system complex assembly, activation by recognition of the tube protein, and inhibition by binding of the DSAD1 protein (Figure 3). They detailed the molecular mechanisms by which the DSR2 system recognizes and activates through the bacteriophage tube protein and how bacteriophages escape the DSR2 immune system by encoding the specific protein DSAD1. These findings deepen our understanding of prokaryotic immune systems, expand our knowledge of the Sir2 domain widely distributed from eukaryotes to prokaryotes, and lay the molecular groundwork for the development of DSR2-based biotechnological tools.
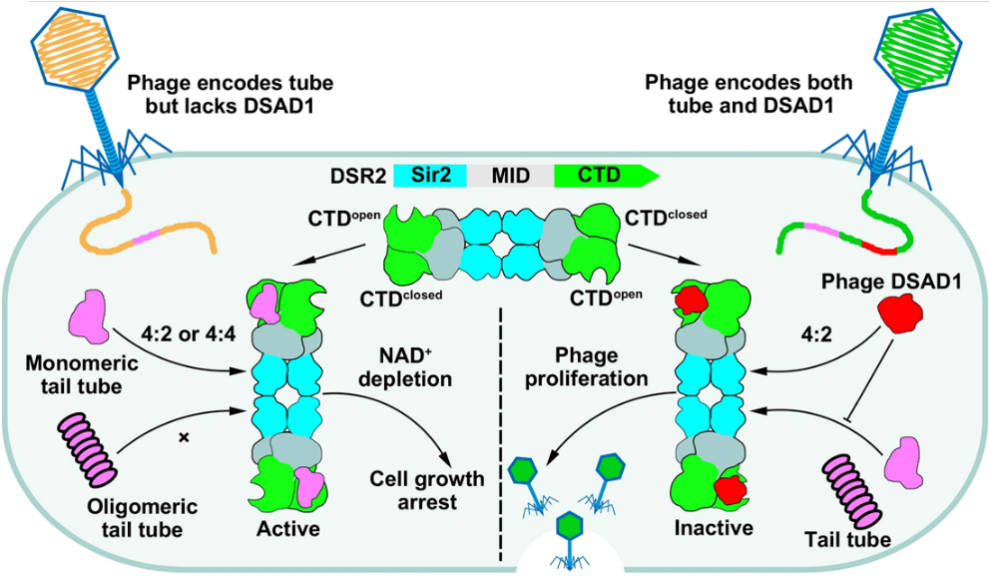
Figure 3. Proposed mechanistic model of prokaryotic DSR2 immune system in response to invading phages
Postdoctoral fellows Jun-Tao Zhang and Xiao-Yu Liu, along with doctoral student Zhuolin Li from the School of Medicine at SUSTech, are the first authors of this paper. Associate Professor Ning Jia is the corresponding author, and SUSTech is the primary affiliation unit.
This work was supported by the National Natural Science Foundation of China (NSFC), Shenzhen and Guangdong Natural Science Foundation, Shenzhen Peacock Program, and the Guangdong Provincial Science and Technology Innovation Council Grant. The researchers also acknowledge the work of the Cryo-EM Center at SUSTech for providing assistance in data collection and data processing.
Paper link: https://doi.org/10.1038/s41467-024-47177-9
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo BySchool of Medicine