Epstein-Barr virus infects more than 90% of adults worldwide, and its infection is associated with multiple diseases, including multiple sclerosis, nasopharyngeal cancer, and B-cell malignancies, posing a significant global health issue. Despite the widespread impact and significant clinical significance of the Epstein-Barr virus, there is currently no vaccine or targeted treatment for Epstein-Barr virus. Understanding the complex mechanisms of Epstein-Barr virus infection and its persistence is still limited.
Epstein-Barr virus surface glycoprotein 42 (gp42) is essential for virus invasion of host B cells. However, the molecular mechanisms by which gp42 interacts with host cells are not fully understood.

Professor Zheng Liu’s research group from the Cryo-Electron Microscopy Center at the Southern University of Science and Technology (SUSTech), in collaboration with Professor Mu-Sheng Zeng from the Sun Yat-sen University Cancer Center (SYSUCC) and Dr. Xiang-Wei Kong from Sun Yat-sen Memorial Hospital, have published their study that identified two neutralizing antibodies and determined the unique binding epitopes, offering insights that facilitate the development of studying EBV neutralizing antibodies and vaccines.
Their research article, entitled “Potent human monoclonal antibodies targeting Epstein-Barr virus gp42 reveal vulnerable sites for virus infection”, has been published in Cell Reports Medicine.
In this study, two strains of gp42 antibody 2C1 and 2B7 were successfully isolated and identified using a phage display library of human antibodies, filling the gap in the international research that has not yet isolated human monoclonal antibodies against EBV gp42.
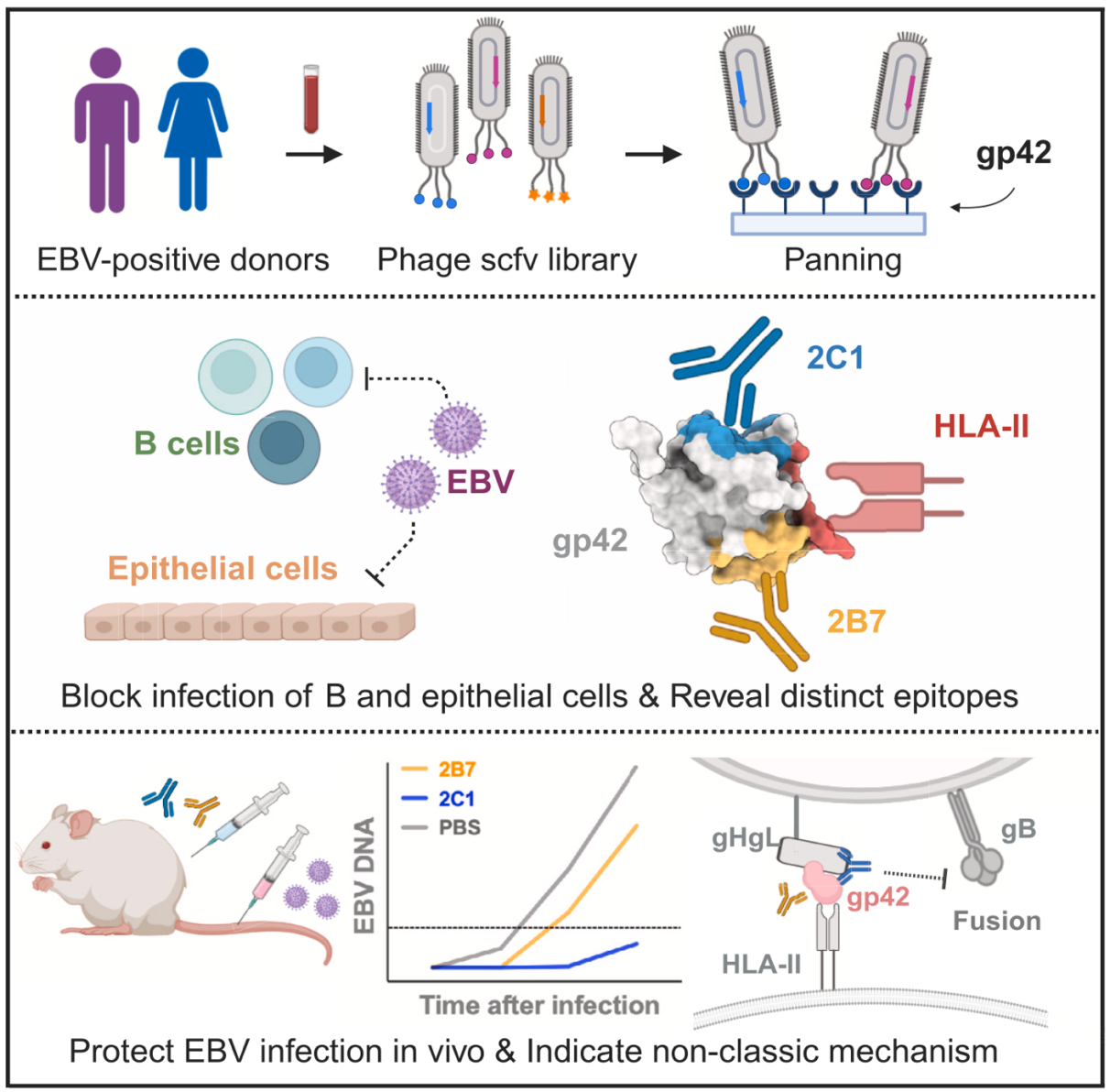
Figure 1. Graphical abstract
This study verified the neutralization ability of the two antibody strains through in vitro and in vivo functional experiments. Combined with structural analysis, it showed that the two antibodies bind to different epitopes on gp42, proposed the neutralization mechanism of anti-gp42 antibodies that block the virus fusion process, and discovered the neutralization effect of gp42 antibodies on epithelial cells.
It provides an important reference for the future design of the EBV vaccine based on gp42. In addition, the study demonstrated that antibody 2C1 is effective in preventing EBV infection in humanized mice, providing a new direction for the development of antibody-based strategies to prevent EBV infection.
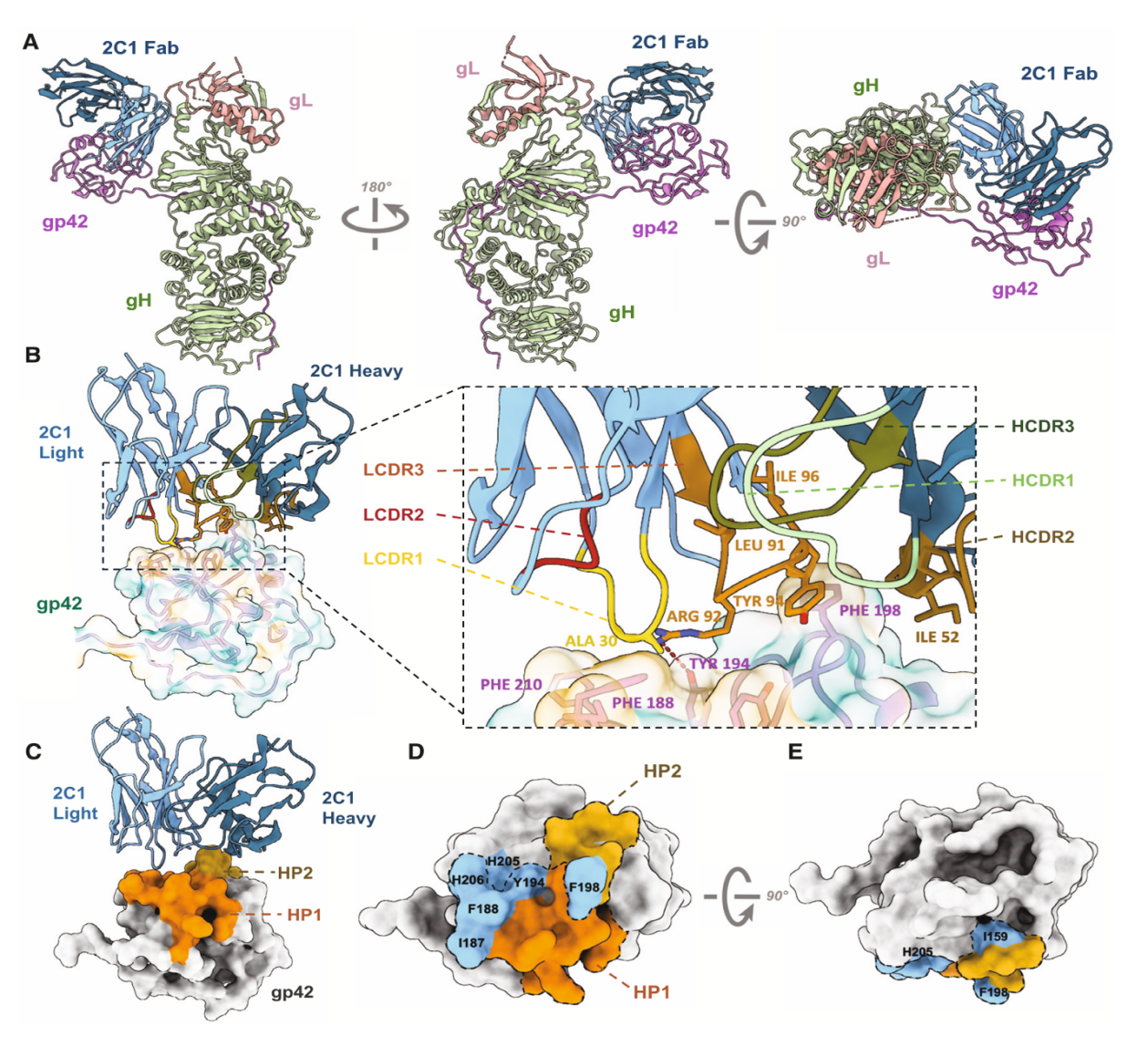
Figure 2. Cryo-EM structure of EBV gH/gL/gp42-2C1 Fab and mutation verification
The structural analysis of the gH/gL/gp42-2C1 and gH/gL/gp42-2B7 complexes was conducted by Prof. Zheng Liu’s group at their Cryo-Electron Microscopy Center. The neutralization epitopes of the antibodies were defined at the molecular level. Analysis of the complex structure of the binding of antibody 2C1 and gp42 showed that the binding site of 2C1 and gp42 was different from the binding site of gp42 and the receptor HLA-II. This structural information provided experimental data support for the antibody neutralization mechanism and provided an important structural basis for the design of the EBV vaccine based on gp42.

Figure 3. 2B7 and 2C1 antibodies reveal two distinct sites on gp42
Ph.D. students Ge-Xin Zhao and Guo-Long Bu from SYSUCC, along with Ph.D. student Xin-Yan Fang from SUSTech, are the co-first authors of the paper. Prof. Mu-Sheng Zeng, Prof. Zheng Liu, and Dr. Xiang-Wei Kong are the co-corresponding authors.
This work was supported by the National Key Research and Development Program of China, National Natural Science Foundation of China (NSFC), Program for Guangdong Introducing Innovative and Entrepreneurial Teams, and the Guangdong Science and Technology Department.
Paper link: https://www.cell.com/cell-reports-medicine/fulltext/S2666-3791(24)00265-9
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread By
Photo ByCryo-Electron Microscopy Center