Small Ubiquitin-like Modifier (SUMO) conjugation, or SUMOylation, is a highly conserved post-translational modification. It is known to regulate the stability, activity, and localization of target proteins and plays a crucial role in all major biological processes in plants. For example, SUMOylation is vital for plant growth and development as mutants lacking the SUMO proteins or key enzymes in the SUMOylation pathway are embryo-lethal. However, due to the relatively low abundance and dynamic nature of SUMOylation and the long SUMO chain remaining after enzymatic cleavage, it has been challenging to identify SUMOylaiton sites in plants, which limits the understanding of SUMOylation’s role and function.

Professor Pengcheng Wang’s lab at the Institute of Advanced Biotechnology, the School of Medicine at the Southern University of Science and Technology (SUSTech) has developed a novel method (K0-SUMO1) that enables, for the first time, to identify SUMOylation modification sites globally in plants. The method, as well as the first site-specific SUMOylation proteomics database in plants, offers a valuable resource for future research.
Their work, entitled “Highly sensitive site-specific SUMOylation proteomics in Arabidopsis”, has been published in Nature Plants.
To address the challenge, Professor Wang’s team expressed a lysine-null variant of SUMO1 (K0-SUMO1) in the Arabidopsis sumo1 sumo2 double mutant. By eliminating lysine from the SUMO protein, K0-SUMO-modified proteins could be more efficiently enriched after enzymatic cleavage. Using an optimized two-step enrichment method, the team conducted a proteomic analysis of Arabidopsis plants expressing K0-SUMO1 under heat stress. It successfully identified over 2,200 SUMOylation sites from more than 1,300 potential SUMOylated proteins, marking the first large-scale identification of SUMOylation sites in plants.
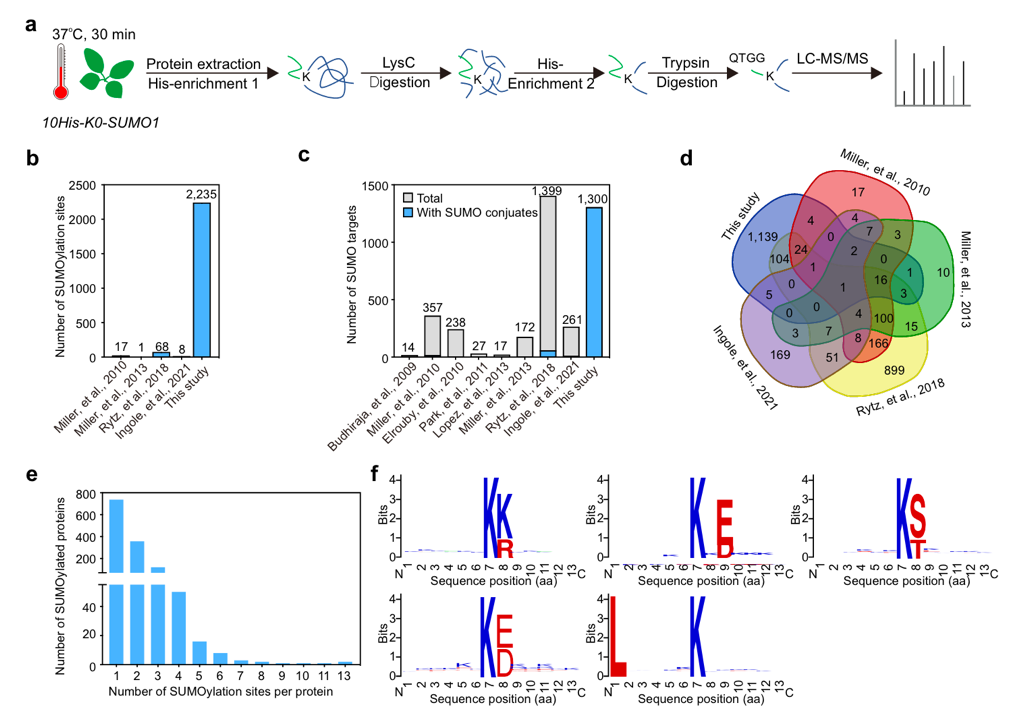
Figure 1. Detection of the Arabidopsis SUMO proteome in K0-SUMO1 transgenic plants
This SUMO proteome revealed that SUMOylation targets are involved in numerous nuclear processes, including gene splicing, gene silencing, chromatin remodeling, DNA repair, and transcriptional regulation. Notably, unlike the classical ψKxE motif in other eukaryotes, SUMOylation in Arabidopsis occurs at multiple motifs, suggesting species-specific differences in the site selectivity of SUMO enzymes.
The team reconstructed the plant SUMOylation system in E.coli. It confirmed that several of the identified sites undergo SUMOylation in vitro, demonstrating the specificity and accuracy of this method. Additionally, quantitative proteomics showed that SUMOylation tended to enhance the stability of substrate proteins under heat-stress conditions.
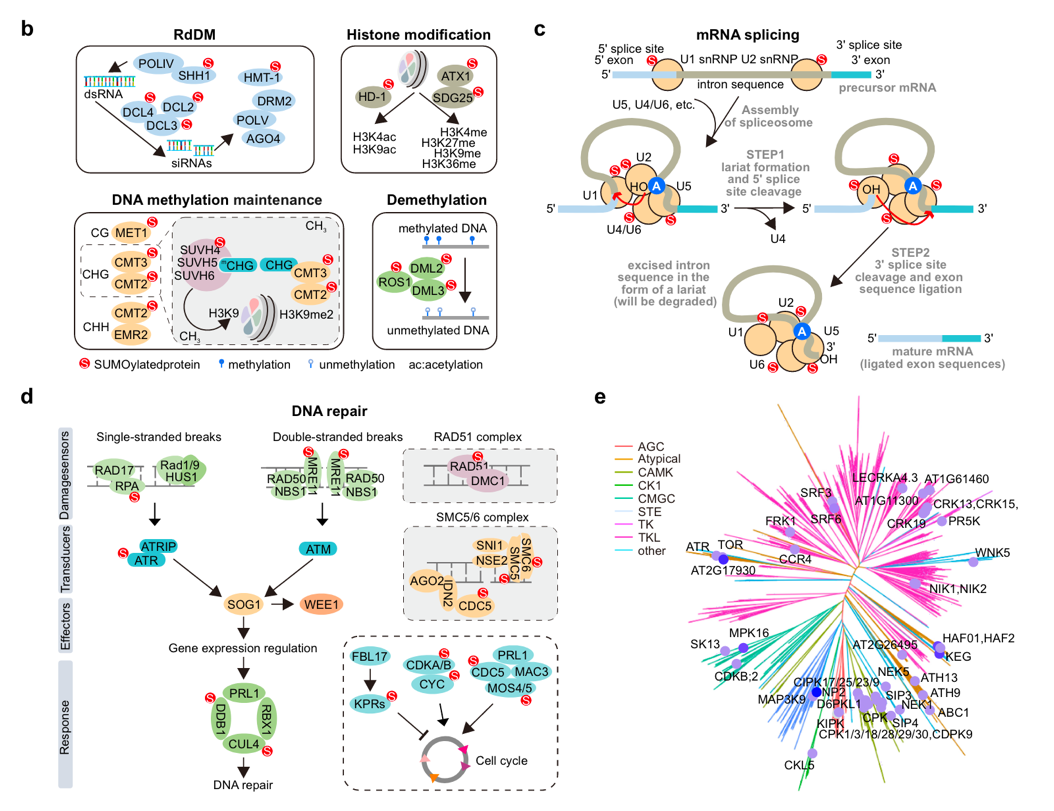
Figure 2. Arabidopsis SUMO target proteins are enriched in nuclear processes
This study represents the first successful large-scale identification of SUMOylation sites in plants at the proteome level. With more than 2,200 modification sites, this work provides a foundation for future research into the functional roles of these modifications through point mutation validation. Moreover, the simple introduction of K0-SUMO could facilitate the identification of SUMOylation substrates and regulatory networks in various plant species, offering a powerful method for investigating key SUMOylation substrates in crops.
Postdoctoral fellow Tian Sang is the first author of the paper. Professor Pengcheng Wang is the corresponding author, and SUSTech is the first affiliated unit. Other contributors to this work include Dr. Shasha Zhao, Dr. Yaping Xu from Anhui Agricultural University, Researcher Guochen Qin from Peking University, and Researcher Quanzhi Xu from the Institute of Plant and Microbial Biology.
The research was supported by the National Key R&D Program.
Paper link: https://www.nature.com/articles/s41477-024-01783-z
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo ByInstitute of Advanced Biotechnology