Living with water, cells are frequently challenged by the imbalance of intra- and extracellular osmotic pressures, which leads to spontaneous water movement across the plasma membrane through a process known as osmosis. For cells, sensing and adapting to these extracellular osmotic alterations is essential to preserve water, maintain cell shape, and enable growth in ever-changing environments. This is particularly true for most plant cells because they are sessile and are directly exposed to the fluctuations of environmental osmolarity.
The increase in soil osmolarity through drought, salinity, and freezing often causes hyperosmotic stress, which leads to rapid water loss, cell shrinkage, metabolic disorders, and stunted growth—posing a major threat to agriculture. Statistics indicate that more than half of global crop production losses are due to osmotic-related abiotic stresses. Consequently, understanding the molecular mechanisms by which plant cells sense and adapt to environmental osmotic stress is of great theoretical value and practical significance.
When cells are exposed to osmotic stress, their volume changes significantly due to the rapid influx or efflux of water. Volumetric change then triggers a series of physical and chemical signals that can be perceived by cells. One established example is that volumetrically turned membrane tension could be perceived by transmembrane mechanosensitive ion channels (including PIEZO, OSCA/TMEM63, and MSL family members), which allow rapid calcium ion influx and thereby initiate intracellular osmotic signal transduction to facilitate stress adaptation. In addition to membrane tension restricted to the plasma membrane, cell volume changes have additional effects spreading all over a cell, such as changing intracellular molecular crowding states. Thus, it raises the question of whether cellular osmosensing could be achieved elsewhere and through distinct mechanisms.
A research team led by Chair Professor Hongwei Guo from the School of Life Sciences at the Southern University of Science and Technology (SUSTech) has revealed a novel mechanism of osmosensing and osmotic stress adaptation in plant cytoplasm mediated by macromolecule crowding-sensitive protein Decapping 5 (DCP5).
Their work, entitled “A cytoplasmic osmosensing mechanism mediated by molecular crowding-sensitive DCP5”, has been published in the journal Science.
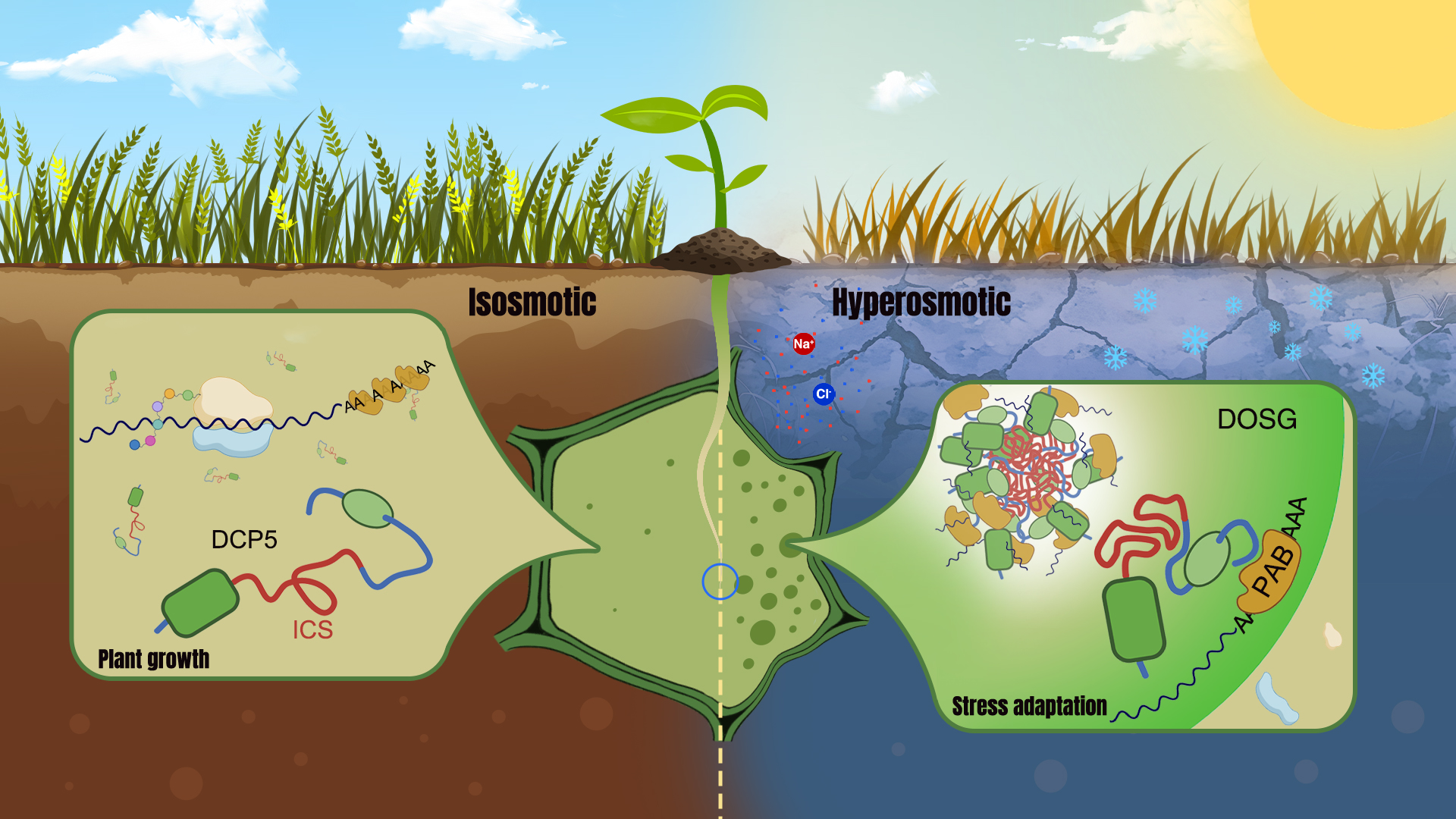
While studying RNA decay-related proteins in Arabidopsis, the researchers found that DCP5 protein rapidly and reversibly assembles to cytoplasmic condensates in response to hyperosmotic stresses. This behavior is independent of the type of stress-triggering osmolytes and is not regulated by canonical osmosignaling pathways. Instead, it is closely correlated with hyperosmotic cell volume changes. DCP5 condenses upon cell shrinkage and returns to a dispersed state during cell volume recovery. Such a rapid and reversible condensation behavior is reminiscent of protein liquid-liquid phase separation (LLPS). They confirmed that DCP5 undergoes LLPS in response to molecular crowding both in vivo and in vitro.

Figure 1. Rapid and reversible condensation of DCP5 in response to hyperosmolarity
The team explored the molecular mechanism underlying osmosensing and phase separation of DCP5. They found that an intrinsically disordered region (IDR) is responsible for the phase behavior of DCP5. When this IDR was deleted, the condensation of DCP5 ceased. The conformation of this IDR also changed as cells became more crowded. Moreover, they found that the IDR of DCP5 is specifically enriched with hydrophobic residues, which provide the driven force of DCP5 phase separation through hydrophobic interactions.
Interestingly, this IDR only appears in DCP5 homologs of land plants. Without this region, other homologs from lower algae, yeast, and animals would not condense upon hyperosmolarity. These results suggest that the IDR in land plant DCP5 may function as an intracellular molecular crowding sensor (ICS) which senses hyperosmolarity through conformational change and triggers phase separation through hydrophobic interactions. This discovery also indicates that the evolution of the DCP5 ICS may help land plant ancestors to adapt to the terrestrial environment.
Through component analysis, the researchers showed that DCP5 condensates are rich in RNA-binding proteins and translation initiation factors, and can recruit a large number of mRNAs through the interaction between DCP5 and polyadenylate-binding proteins (PABs). This newly identified structure, termed the DCP5-enriched osmotic stress granule (DOSG), significantly changes the translation efficiency of many genes due to the isolation of mRNA and translation factors as it assembles. In addition, by sequestration of some transcription factors and nucleocytoplasmic transport proteins, DOSG also reshapes the transcriptome. In the absence of DCP5, plants were hypersensitive to hyperosmotic stress and could not be fully rescued by DCP5 mutant protein lacking the ICS.
These results indicate that through phase separation and DOSG assembly, DCP5 not only senses osmotic stress, but also directly mediates stress responses at the translational and transcriptional levels to facilitate plant stress adaptation.

Figure 2. Schematic diagram of osmosensing and stress adaptation mediated by DCP5
This work uncovers a cytoplasmic osmosensing and stress adaptation mechanism mediated by the molecular crowding–triggered phase separation of DCP5 and DOSG assembly. It identifies DCP5 as a multifunctional osmosensor that participates in both osmosensing and osmotic stress adaptation, and suggested a stress sensory function for hyperosmotically induced stress granules.
Research Assistant Professor Zhenyu Wang and Chair Professor Hongwei Guo from SUSTech are the first and corresponding authors of this paper, respectively. Additional co-authors from SUSTech include doctoral student Qiuhua Yang; graduate students Yuanyi Lu and Ruixue Sun; Drs. Dan Zhang, Yichuan Wang, Yajie Pan, Yuping Qiu, Wei Yan, and Wenyang Li; Research Scholar Zhina Xiao; Associate Professor Hongda Huang; and Researcher Yongfan Men from Shenzhen Institutes of Advanced Technology (SIAT) of the Chinese Academy of Science (CAS).
Paper link: https://www.science.org/doi/10.1126/science.adk9067
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo BySchool of Life Sciences