A research team led by Chair Professor Haiping Xia from the Department of Chemistry and Executive Dean of the Shenzhen Grubbs Institute at the Southern University of Science and Technology (SUSTech) has recently made a groundbreaking achievement in annulene chemistry. For the first time, the team successfully embedded a metal atom at the center of an annulene plane, synthesizing an aromatic metal-centered [15]annulene. This milestone breakthrough addresses a 70-year gap in the field of metallo-annulenes, expanding the frontiers of annulene chemistry, aromatic chemistry, and organometallic chemistry.
Their work, titled “Metal-centered planar [15]annulenes”, has been published in Nature. An article was also published in the News & Views segment of the journal, entitled “Metal-cored molecule is the first of its kind”. Additionally, it was highlighted by C&EN News, titled “Osmium ensnared in a hydrocarbon ring”.

Annulene chemistry: From benzene to ferrocene, a two-century journey
Annulenes, fully conjugated polyenes, epitomize the evolution of modern chemistry. The journey began in 1825, Michael Faraday discovered benzene (Figure 1a), the first known annulene and the cornerstone of aromatic chemistry. Over a century later, in 1951, the discovery of ferrocene (Figure 1b)—a sandwich complex with metals outside the annulene plane—revolutionized organometallic chemistry. However, for the past 70 years, scientists struggled to place metals within annulene planes due to structural constraints (small rings like benzene lack space; medium/large rings are non-planar) and activation challenges (lack of directing groups for C-H bond activation). Efforts to embed metals in “all-carbon porphyrins” (Figure 1c), another holy grail of porphyrin chemistry, remain exploratory.
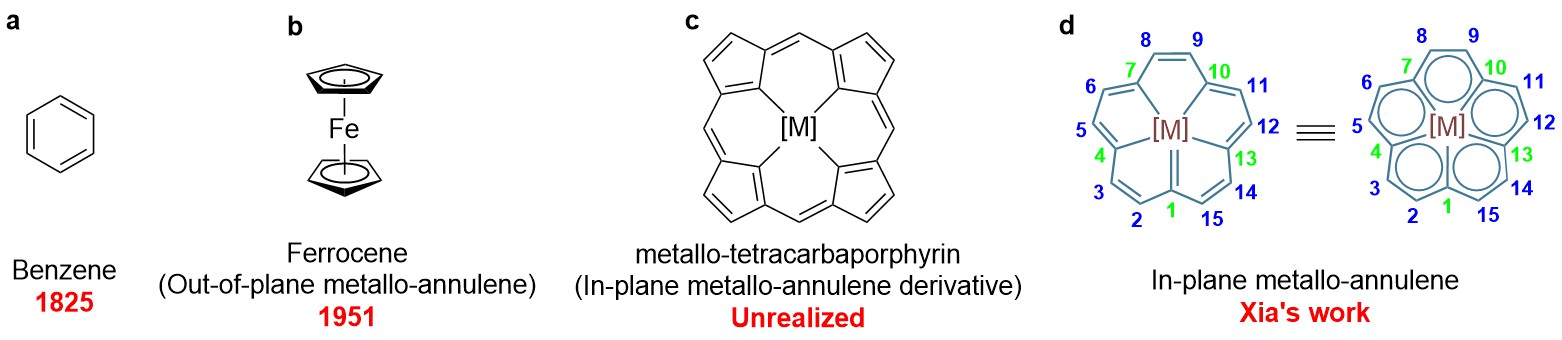
Figure 1. Evolution of annulene chemistry: (a) Benzene, the first annulene; (b) Ferrocene, an out-of-plane metallo-annulene; (c) Hypothetical metallo-tetracarbaporphyrin; (d) In-plane metallo-[15]annulene achieved in Xia’s work
Breakthrough strategy: Carbolong chemistry enables planar metallo-annulenes
Professor Xia’s team, widely renowned for the original “Carbolong Chemistry” (Figure 2), has continued to push the boundaries of molecular design. This unique chemistry has been recognized in March’s Advanced Organic Chemistry (8th ed.), and earned the Second Prize of China’s National Natural Science Award in 2020.
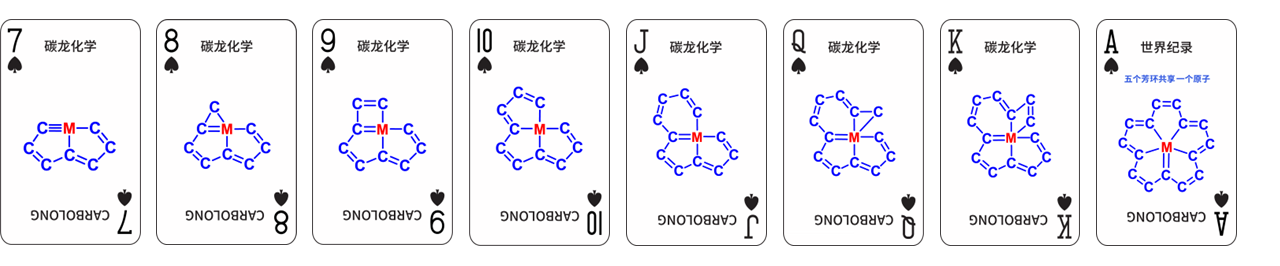
Figure 2. The “Carbolong” system
In their latest work, the researchers designed a cycloaddition strategy between carbolong complexes and an alkyne as the key step, affording a planar D5h-symmetric metallo-[15]annulene fragment (Figure 3).
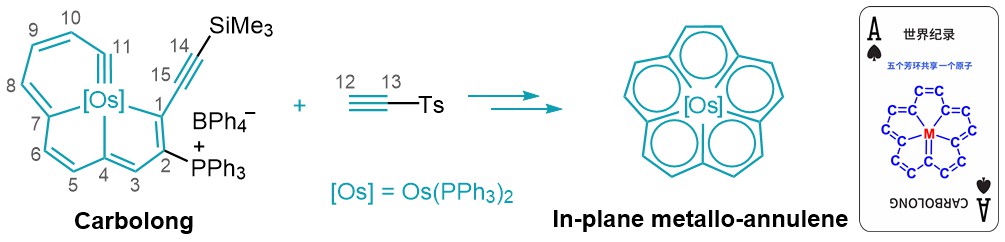
Figure 3. Cycloaddition strategy for the synthesis of in-plane metallo-[15]annulene
Experimental and computational analyses confirmed the annulene’s aromaticity rivals benzene: 1). ¹H NMR: 10 protons at 9.43 ppm (vs. benzene’s 7.36 ppm); 2). NICS(1)zz: -41.9 ppm (vs. benzene’s -29.3 ppm); 3). ASE: 93.3 kcal/mol (vs. benzene’s 33.1 kcal/mol).
Properties and applications: From fundamentals to advanced materials
The planar metallo-annulene exhibits robust stability, remaining air-stable at 240°C, and showing reversible electrochemistry behavior akin to ferrocene. It also features a unique photoresponse, displaying aggregation-induced emission (AIE) with visible red light under UV irradiation. In addition, the molecule offers versatile derivatization, undergoing nitration, iodination, and full-chlorination in its annulene plane, as well as axial ligand substitution (Figure 4), positioning it as a building block for two- and three-dimensional materials.
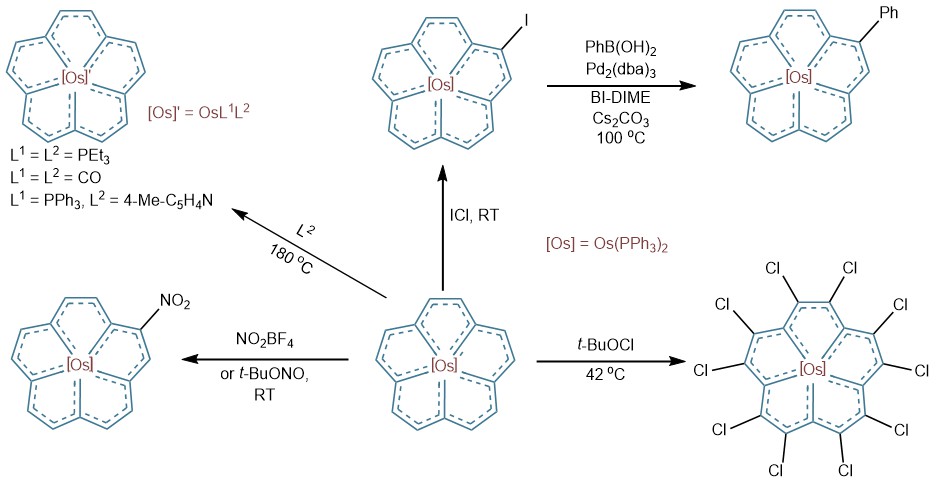
Figure 4. Derivatization reactions of in-plane metallo-[15]annulene
Interdisciplinary convergence: Bridging annulene and porphyrin chemistry, while integration organic and inorganic chemistry
The molecule embodies both scientific and aesthetic elements (Figure 5): Structurally, it resembles metallo-porphyrins and can be viewed as a nitrogen-removing analog; it also mirrors the architecture of corannulene—a C60 fragment)—and can be viewed as the structure by replacing the inner five-membered ring of corannulene with a metal. Culturally, its fivefold symmetry mimics the plum blossom, one of China’s “Ten Great Flowers”, and the logo of the Department of Chemistry at SUSTech.
To reflect both its compositional identity and aesthetic form, the team has proposed the name “Carborin”—blending Carbon, porphyrin, and ring—to honor its scientific essence and elegance.
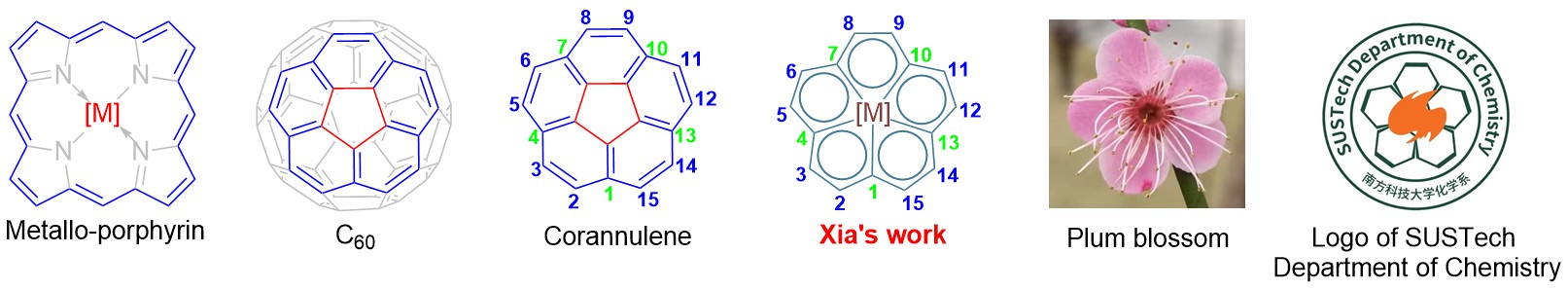
Figure 5. In-plane metallo-[15]annulene and structural comparisons
Postdoctoral fellows Binbin Xu and Kaidong Ruan, Research Professor Dafa Chen, and Assistant Research Professor Ming Luo—all from SUSTech—are the co-first authors of the paper. Chair Professor Haiping Xia is the sole corresponding author, with SUSTech serving as the primary affiliated institution. Other contributors to this work from SUSTech include postdoctoral fellow Yuanting Cai, master’s students Jia Qiu and Wenhao Zhou, and Dr. Bula Cao, along with Chair Professor Zhenyang Lin from The Hong Kong University of Science and Technology and Professor Jonathan L. Sessler from The University of Texas at Austin.
Paper link: https://www.nature.com/articles/s41586-025-08841-2
News & Views: https://www.nature.com/articles/d41586-025-01135-7
C&EN News: https://cen.acs.org/synthesis/Osmium-ensnared-hydrocarbon-ring/103/web/2025/05
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yilin ZHOU
Photo ByYan QIU