As the number of COVID-19 infections continues to grow, an increasing number of patients are suffering from “Long COVID”. Unlike the severe symptoms during the acute phase, Long COVID generally refers to chronic post-COVID conditions, encompassing a wide range of long-term symptoms experienced after recovering from the infection.
Multiple epidemiological studies indicate that the prevalence of Long COVID is around 12-17% among infected individuals. Conservatively estimated, the global number of Long COVID patients has approached 100 million. Apart from the respiratory system, the cardiovascular system is one of the most severely affected by Long COVID, with common disorders including arrhythmias, hypotension, venous thromboembolism, myocarditis, and heart failure. As the COVID-19 pandemic enters a phase of fluctuating prevalence, the pathological mechanisms underlying Long COVID-related cardiovascular manifestations require urgent clarification.
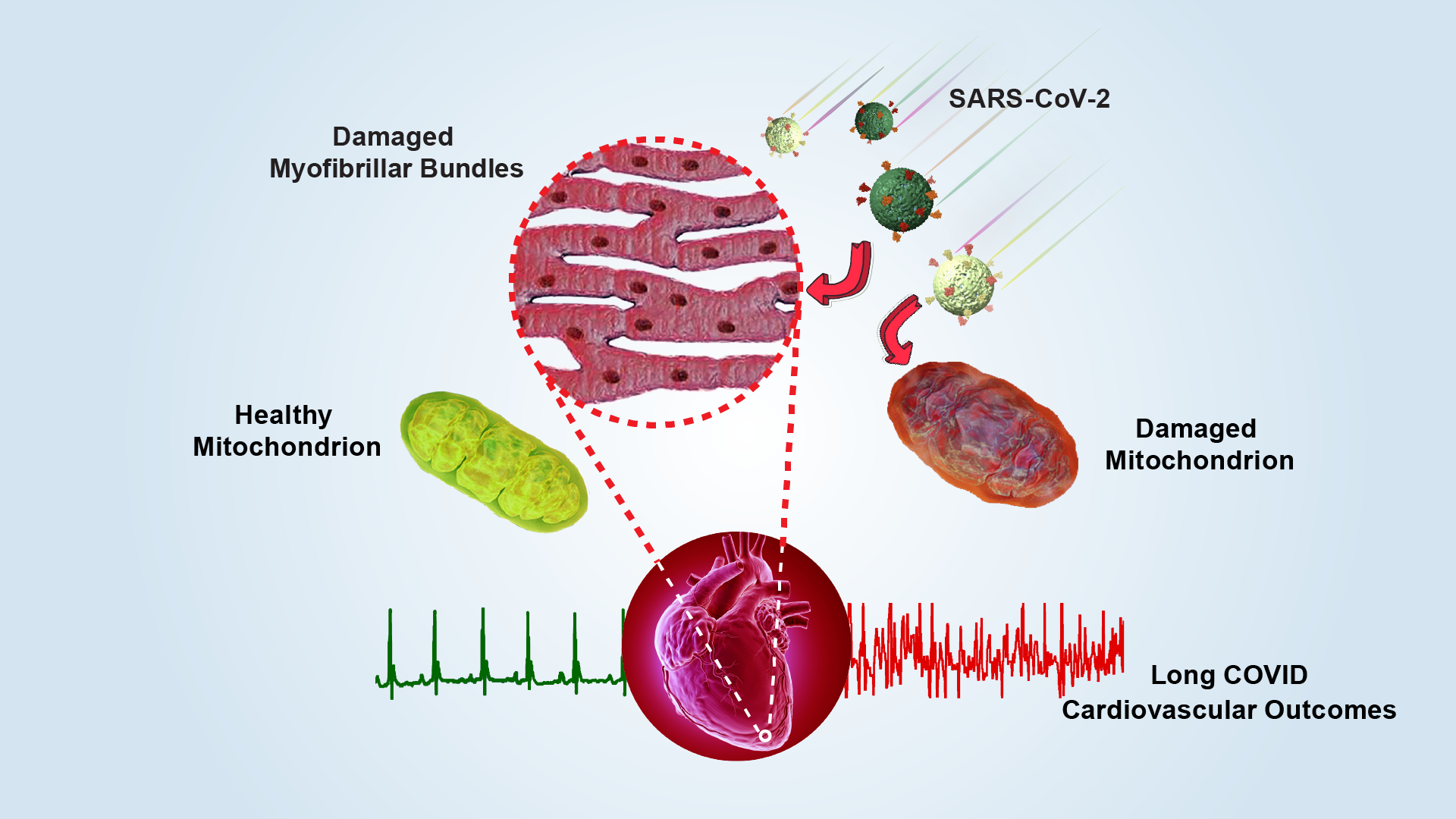
A research team led by Professors Zheng Liu and Sen-Fang Sui from the Cryo-Electron Microscopy Center (Cryo-EM Center) and the School of Life Sciences at the Southern University of Science and Technology (SUSTech), in collaboration with Professors Yawei Xu and Wenliang Che’s team from the Department of Cardiology at Shanghai Tenth People’s Hospital, Tongji University School of Medicine, and Professors Jincong Zhao and Yanqun Wang’s team from the State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease at The First Affiliated Hospital of Guangzhou Medical University, has systematically revealed the long-term damage to mitochondria in cardiomyocytes caused by SARS-CoV-2 infection. Their work provides direct cellular evidence to explain the pathogenesis of cardiovascular diseases associated with Long COVID.
Their paper, entitled “SARS-CoV-2 damages cardiomyocyte mitochondria and implicates long COVID-associated cardiovascular manifestations”, has been published in the Journal of Advanced Research.
The study focused on five clinical Long COVID patients who developed various cardiovascular symptoms, including syncope, cardiac arrest, palpitations, chest pain, shortness of breath, and fatigue within one to three months after infection. Endomyocardial biopsies were obtained from these patients, and histopathological, immunohistochemical, and electron microscopy (EM) features of myocarditis were observed in all patients, such as inflammatory cell infiltration, myofibril bundle damage, and interstitial fibrosis.
Further EM analysis showed widespread mitochondrial structural abnormalities in the cardiomyocytes: a significant number of mitochondria were swollen and vacuolated, with distorted and broken cristae. Structural damage to mitochondria disrupts ATP synthesis, resulting in an insufficient energy supply required for proper cardiac contraction. This energy deficit can trigger a range of cardiac disorders.
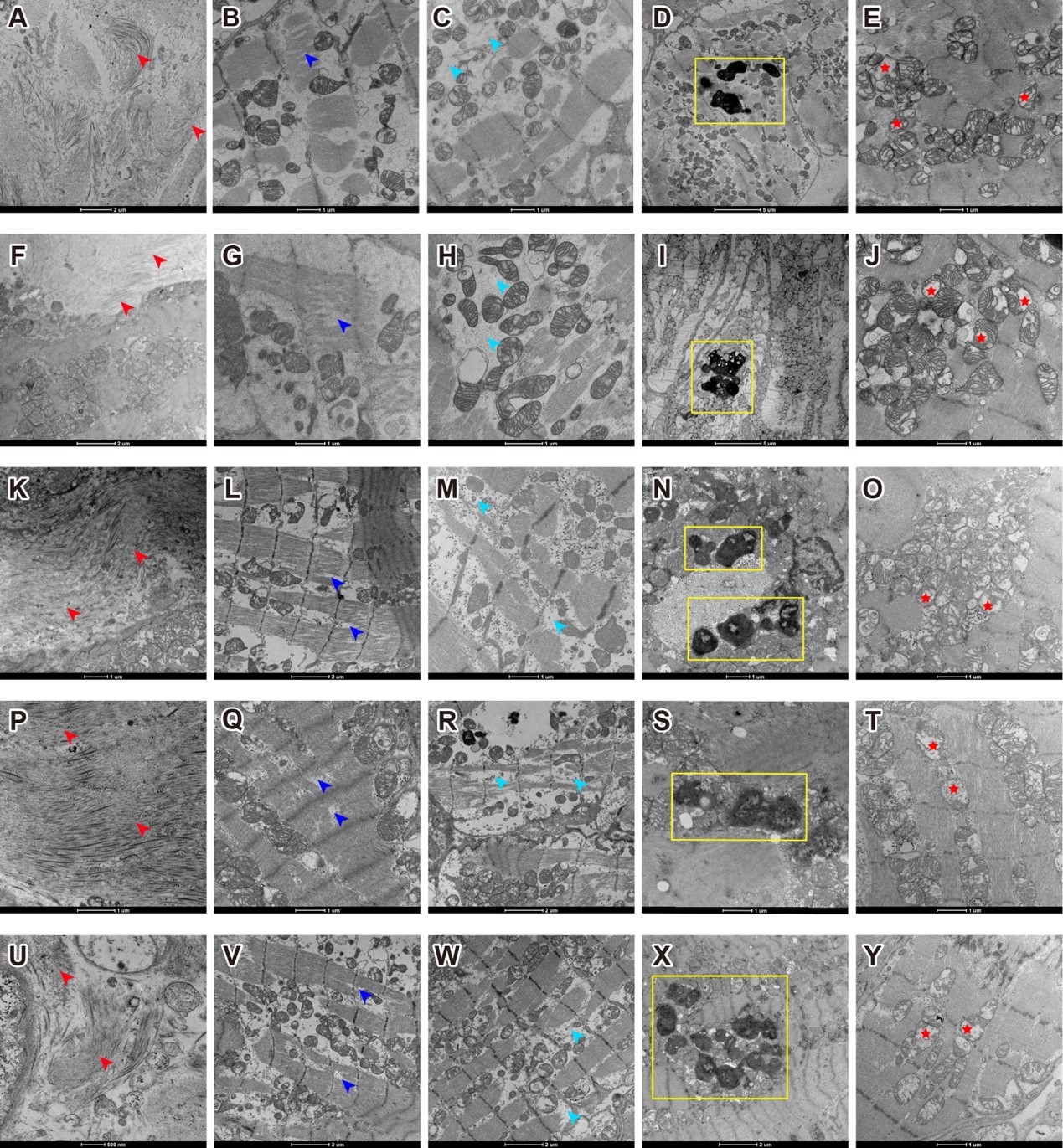
Figure 1. Electron microscopy of myocardial biopsy tissue samples from five Long COVID patients shows fibrosis accumulation, myofibril bundle damage and necrosis, lipofuscin accumulation, and severe mitochondrial structural damage
To further quantify mitochondrial ultrastructural damage, the researchers employed focused ion beam-scanning electron microscopy (FIB-SEM) to perform three-dimensional volume EM of the biopsy samples. By continuously slicing the tissue with an ion beam (each layer with a thickness of 10 nanometers) and collecting image data, they constructed a micrometer-scale 3D structural model of the tissue block.
The team developed a deep-learning software, MitoStructSeg, to automatically segment the mitochondrial outer membrane, inner membrane, and cristae. Results showed that 60% to 90% of the mitochondria exhibited structural disorder, significantly higher than the 40% to 60% estimated from 2D images, indicating that 3D analysis reveals mitochondrial damage more precisely than traditional 2D imaging. By calculating cristae density (SCRIS/VMITO), the study suggested severe impairment of energy metabolism functions. This 3D quantitative method overcomes biases introduced by the unidirectional slicing of traditional EM sections, providing higher-resolution and more accurate assessments for mitochondrial pathology analysis.
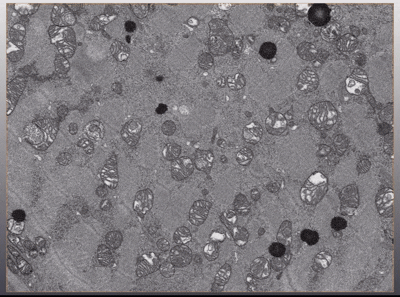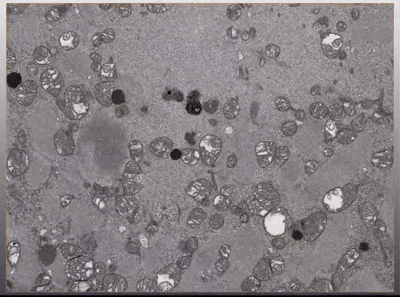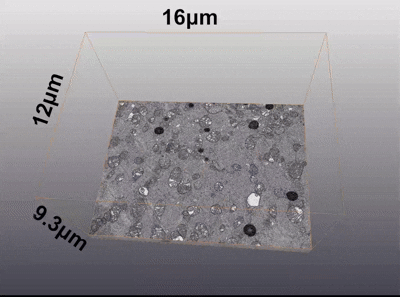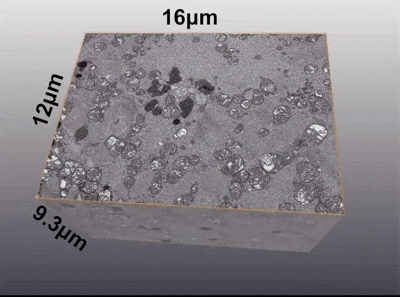
Animation 1. 3D volume electron microscopy of myocardial biopsy tissue samples
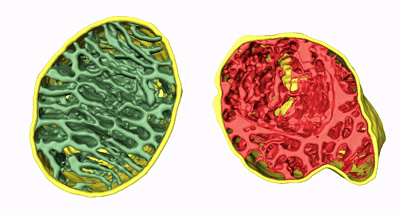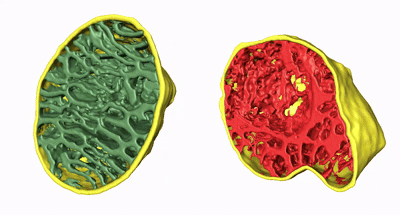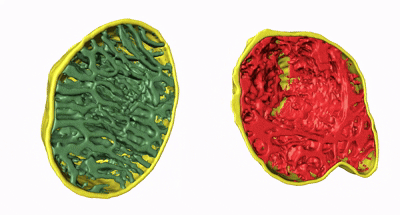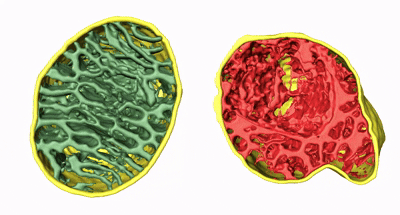
Animation 2. 3D structure comparison between healthy and damaged mitochondria
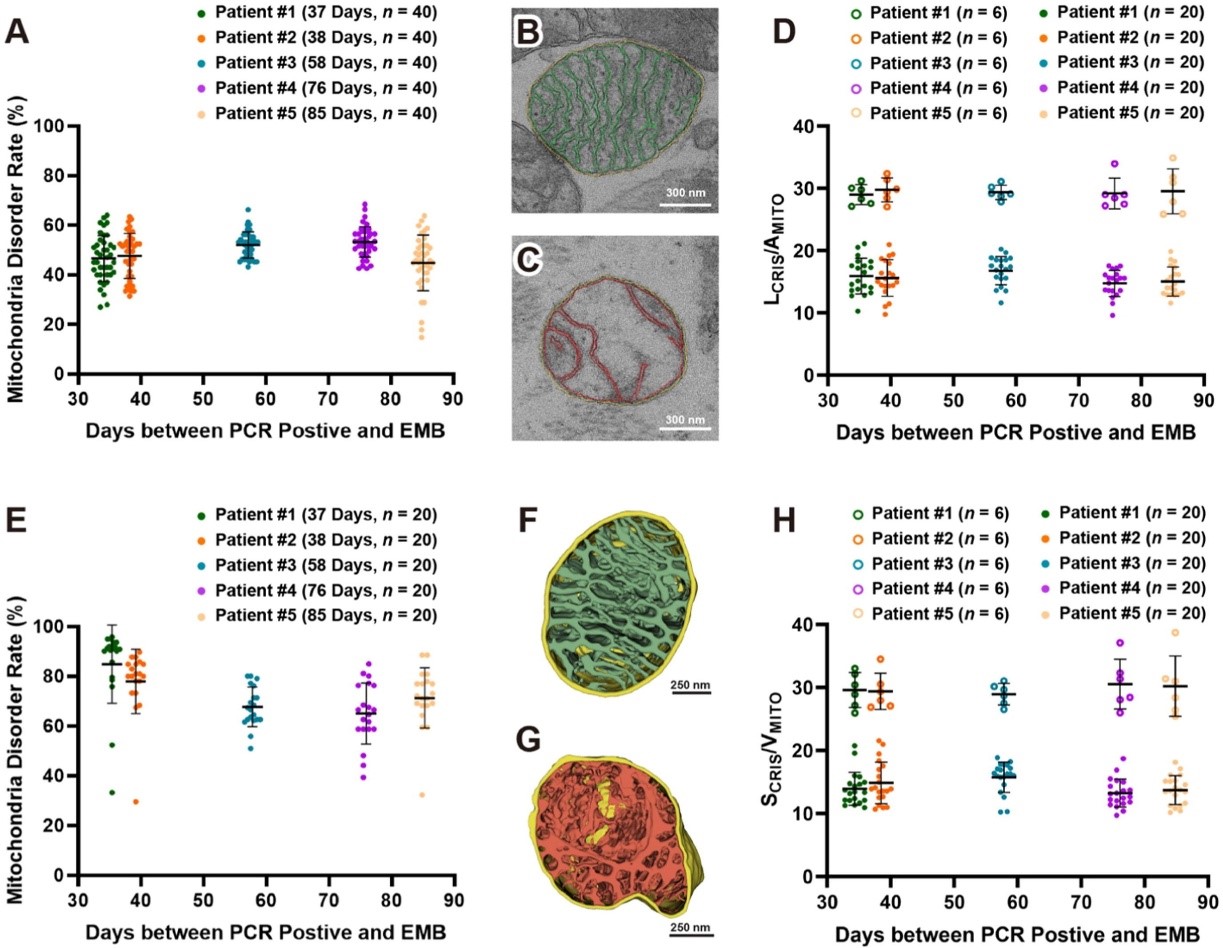
Figure 2. 3D qualitative and quantitative analysis of mitochondrial damage in five patients
In an animal study, cardiac tissue from mice infected with SARS-CoV-2 Omicron sublineages BA.5.2 and BQ.1 also showed mitochondrial ultrastructural abnormalities. EM results indicated that the infected mice’s cardiomyocyte mitochondria exhibited cristae fragmentation, loose arrangement, and in some cases, swelling and vacuolization of the mitochondrial matrix—highly consistent with the pathological characteristics observed in human cases.
Proteomics profiling revealed significant changes in mitochondrial-associated proteins, which were primarily enriched in key pathways including mitochondrial oxidative phosphorylation, mitochondrial outer and inner membrane protein transports, mitochondrial structural maintenance proteins, and cellular self-protection proteins. Notably, upregulated mitochondrial-related proteins included transporters (TOM40, TOM20, TIM13, and the ADP/ATP carrier protein Slc25a13), which compensate for membrane damage by enhancing precursor protein input. Other upregulated proteins included mitochondrial homeostasis regulators (such as the outer membrane sorting and assembly component Samm50, the mitochondrial fragmentation inhibitor Prohibitin 1, and the mitochondrial inner membrane protein Mpv17), as well as components of oxidative phosphorylation complexes (cytochrome c oxidase, cytochrome bc1 complex, and NADH dehydrogenase). These findings further explain the molecular mechanisms by which SARS-CoV-2-induced mitochondrial damage triggers various cardiovascular diseases.
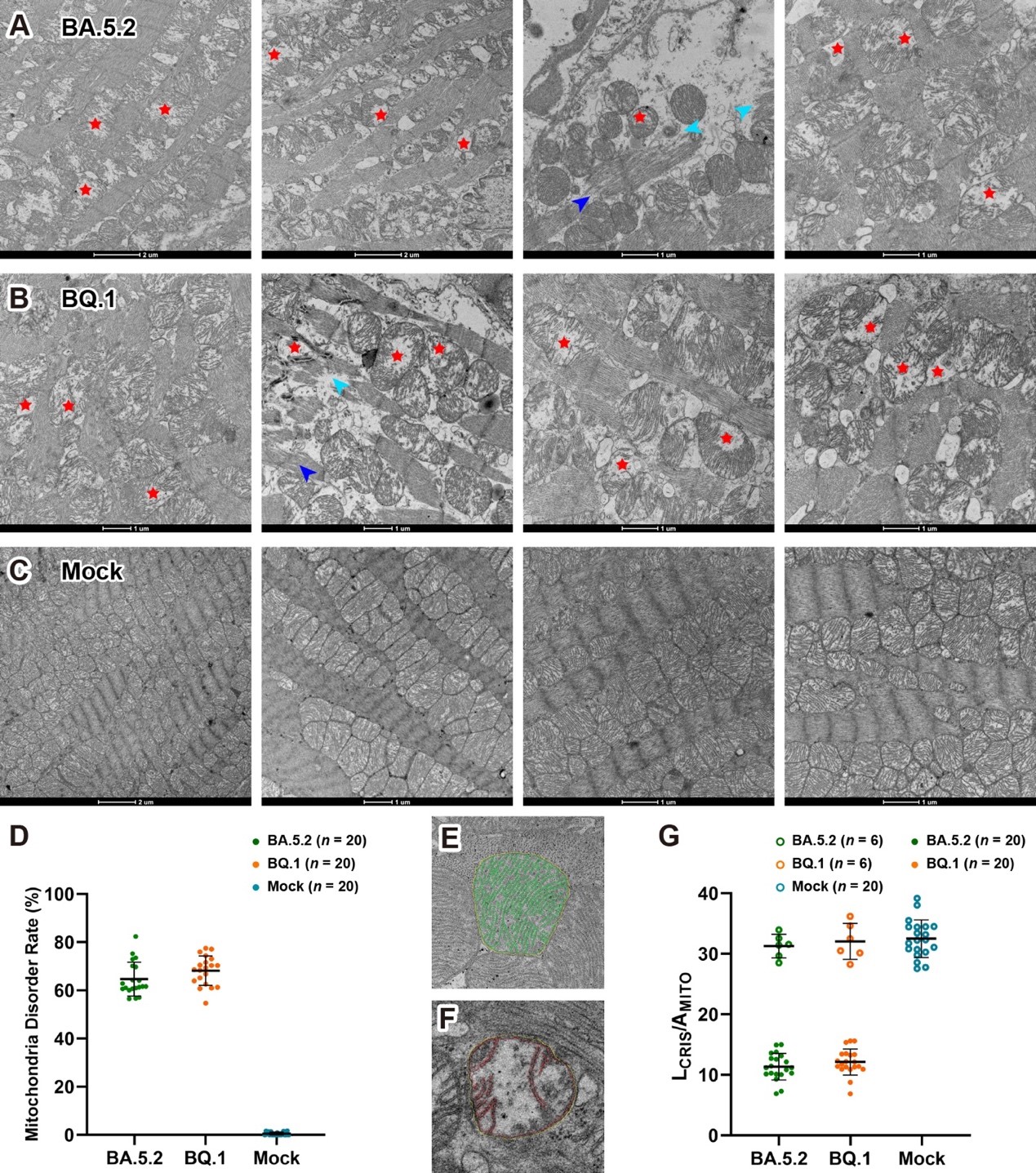
Figure 3. Mitochondria disorganization in SARS-CoV-2 infected mice
By combining clinical data with animal models and using 3D volume electron microscopy and proteomics analysis, this study clarified the pathological features of the mitochondrial cristae system and uncovered how host cells regulate cellular stress response through mitochondrial homeostasis. This deepens the understanding of the multi-system impact of SARS-CoV-2 infection and lays the theoretical foundation for developing therapeutic strategies targeting mitochondrial dysfunction.
Professor Wenliang Che, Ph.D. student Shuai Guo from the School of Life Sciences at SUSTech, and Professor Yanqun Wang are the co-first authors of the paper. Professors Zheng Liu, Yawei Xu, Jincong Zhao, and Sen-Fang Sui are the corresponding authors. Other contributors to this work include Professors Xiaohua Wan and Fa Zhang from the Beijing Institute of Technology, and Professors Peiyi Wang and Jian Zhu from the Cryo-EM Center at SUSTech.
Paper link: https://doi.org/10.1016/j.jare.2025.05.013
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yuwen ZENG
Photo BySchool of Life Sciences