Glycogen, an intricate glucose polymer exhibiting a highly branched structure, serves as a crucial energy reservoir in numerous animals, including humans. Predominantly stored in the liver and muscle cells, glycogen is capable of being promptly mobilized to satisfy the body’s energy demands and to maintain stable blood glucose levels during periods of intense activity or fasting. Beyond these primary storage sites, it has also been detected in other tissues such as the brain, heart, and kidney.
In the human body, the breakdown of glycogen primarily occurs within the cytoplasm, though a minor fraction of glycogen degradation is mediated by α-glucosidase within lysosomes. The glycogen degradation in the cytoplasm involves a synergistic action between two enzymes: glycogen phosphorylase (GP) and glycogen debranching enzyme (GDE). Through their collaborative efforts, glycogen is converted into glucose and glucose-1-phosphate, thereby stabilizing blood glucose levels and providing essential energy.
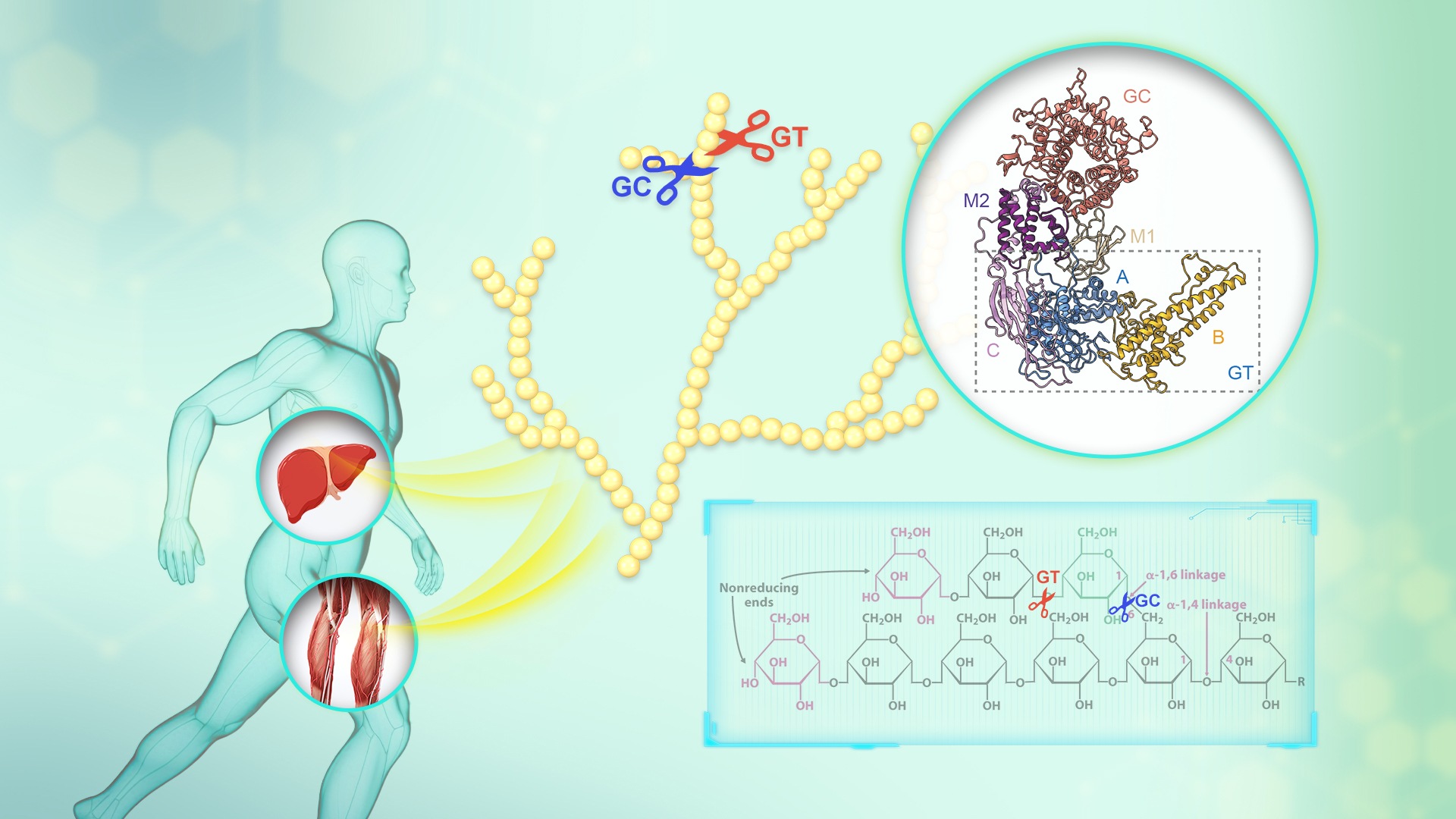
GDE occupies a pivotal role in glycogen degradation. The glucose residues of glycogen are connected through α-1,4 glycosidic bonds, with branch points being linked by α-1,6 glycosidic bonds. GP sequentially removes glycosyl units from the non-reducing terminus of glycogen, generating limit dextrin and glucose-1-phosphate. Subsequently, GDE, a bifunctional enzyme possessing both 4-alpha-glucanotransferase (GT) and amylo-α-1,6-glucosidase (GC) activities, eliminates the branches of limit dextrin in two steps.
Initially, the GT activity transfers the maltotriosyl group, linked by α-1,4 glycosidic bonds, from the branch to a proximate non-reducing end of glycogen. Next, the GC activity hydrolyzes the α-1,6 glycosidic bonds at branch points, catalyzing the removal of glucose residues and releasing free glucose. Dysfunctional GDE causes glycogen storage disease type III (GSD III), a complex genetic disorder with multi-organ complications.
A research team led by Assistant Professor Kaige Yan from the Departments of Chemistry and Biology, School of Life Sciences at the Southern University of Science and Technology (SUSTech) has uncovered the molecular architecture of human GDE. Their study not only advances the understanding of hsGDE’s role in substrate recognition and catalysis but also illuminates the molecular pathology of GSD III, which will offer the development of targeted therapeutic strategies for this disease.
Their work, “Molecular Architecture and Catalytic Mechanism of Human Glycogen Debranching Enzyme,” has been published in the journal Nature Communications.
The team successfully determined the high-resolution cryo-EM structure of human GDE. They revealed a unique domain arrangement of GDE where GT and GC catalytic sites reside at opposite ends of the protein, connected by two middle domains. This configuration optimizes both stability and functional flexibility during glycogen degradation.
They performed molecular dynamics simulations and demonstrated how GDE selectively recognizes glycogen. The GT domain’s binding pocket uses hydrophobic and charged residues to stabilize substrate interactions, while its B-domain acts as a molecular “clamp” that adjusts conformation to accommodate glucose chains and enhance transfer efficiency.
The researchers identified key residues in GT/GC catalytic pockets essential for enzymatic activity through conservation analysis, validated by mutagenesis studies. Finally, they found that GSDIII-related mutations in catalytic residues directly impair enzymatic activity, whereas non-catalytic mutations disrupt structural integrity or substrate binding—explaining GSD III’s clinical heterogeneity.
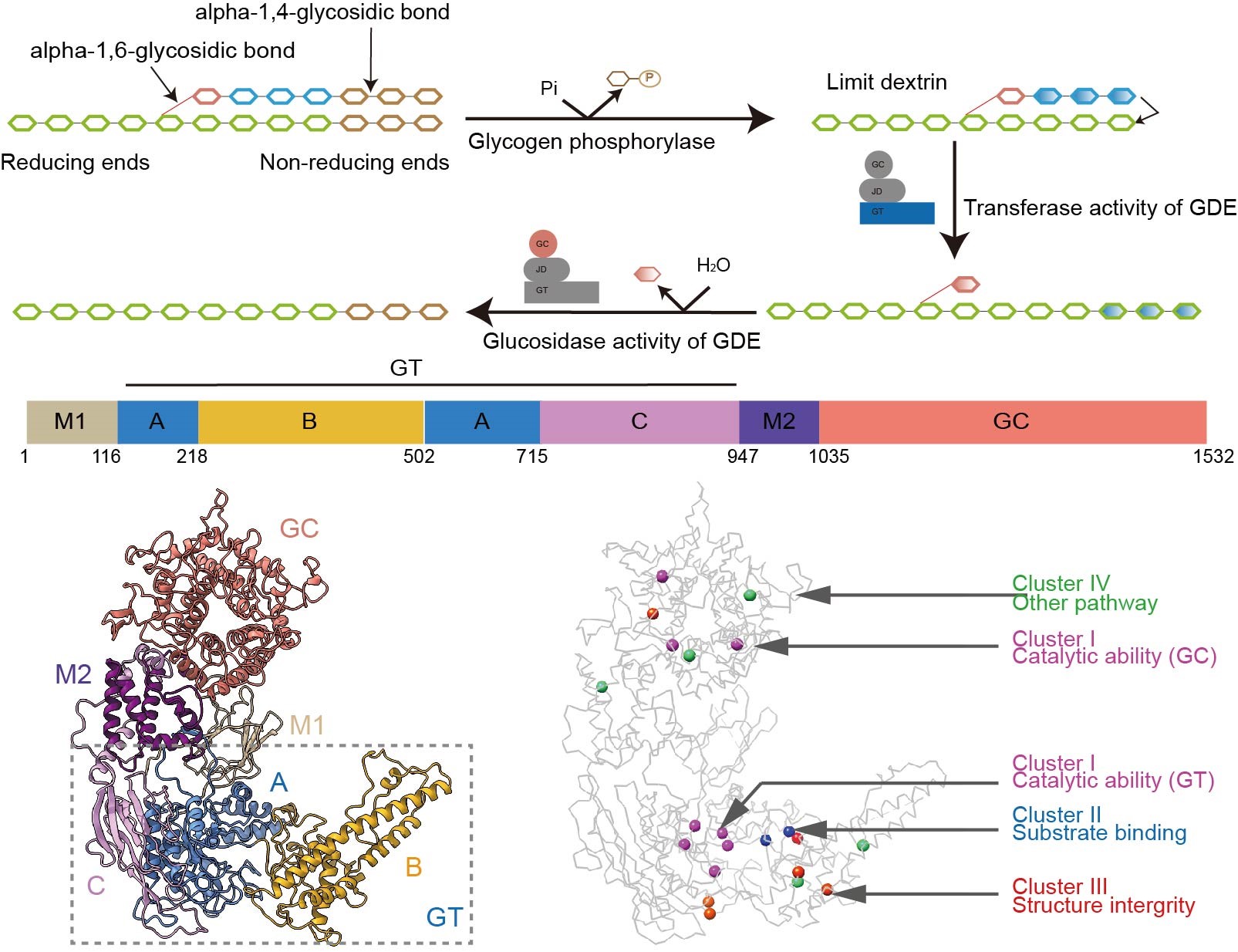
Figure 1. The structure of human GDE and the pathogenic mechanism of GSDIII
Master’s graduate Huiyi Guan, Ph.D. graduate Huan Chen, and master’s student He Geng from the School of Life Sciences at SUSTech are the co-first authors of the paper. Assistant Professor Kaige Yan and former Research Assistant Yifang Chen from Zhejiang University serve as the corresponding authors. Additional contributors to this work include Professor Yong Wang from Zhejiang University, along with Research Assistant Professor Ruifang Ma and Assistant Professor Zhongmin Liu from SUSTech.
Paper link: https://www.nature.com/articles/s41467-025-61077-6
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yifei REN
Photo BySchool of Life Sciences