Colorectal cancer (CRC) poses a major global health challenge due to its high incidence and accelerated increase, particularly the surge in early-onset cases. Environmental factors serve as primary drivers, with their core pathological impact lying in disrupting the intestinal epithelial barrier and thereby leading to inflammatory bowel disease (IBD). IBD patients face dramatically elevated CRC risk, as barrier breach triggers pro-inflammatory feedback that establishes a vicious cycle. However, how inflammation precisely controls the dynamics of intestinal barrier, particularly the E-cadherin based adherens junction, and how this drives cancer development, is still unclear.
Researchers at the Southern University of Science and Technology (SUSTech) have elucidated a mechanism by which environmental stimuli disrupt the intestinal epithelial barrier and drive inflammation-associated carcinogenesis. This mechanism promotes tumor progression by inducing epithelial-mesenchymal transition (EMT) and activating the Wnt signaling pathway, thereby cooperatively enhancing tumor cell proliferation and migration. These findings provide new insights into the significant rise in colorectal cancer incidence observed in recent years, while identifying a new signaling pathway as an actionable target for intervention to suppress inflammation-driven cancer development, laying the groundwork for novel prevention strategies.
Professor Feng Rao’s team from the School of Life Sciences at SUSTech has long been dedicated to deciphering the emerging signaling functions of inositol polyphosphates. In their recent study, published in Nature Chemical Biology under the title “Oncometabolite 5-IP7 inhibits inositol 5-phosphatase to license E-cadherin endocytosis,” they focused on 5-diphosphoinositol pentakisphosphate (5-IP7), a high-energy signaling molecule generated by IP6K2-mediated phosphorylation of IP6. Their prior work pioneered the discovery that IP6 acts as a metabolic modulator of Cullin-RING E3 ubiquitin ligases (CRLs) in glucose-stimulated insulin secretion (Nature Communications, 2021), followed by the revelation that 5-IP7 regulates insulin vesicle release as a neurotransmitter GPCR messenger (Nature Metabolism, 2021). This latest work marks their continued and deepened exploration into phosphoinositide signaling mechanisms.
The team identified a novel oncogenic metabolite, 5-IP7, which drives PI(4,5)P2-dependent E-cadherin endocytosis by inhibiting inositol 5-phosphatases that degrades PI(4,5)P2. This disrupts the intestinal epithelial barrier and activates the β-catenin oncogenic pathway, promoting the development of colorectal cancer. This work provides a new therapeutic target and validates inhibitors, offering a critical breakthrough for curbing inflammation-driven colorectal cancer.
This study revealed through clinical sample analysis that IP6K2 is significantly overexpressed in colorectal cancer tissues and negatively correlates with patient 5-year survival rates. To elucidate its pro-tumorigenic mechanism, the researchers established intestinal epithelium-specific IP6K2 knockout and kinase-dead mutant mouse models. These models demonstrated that IP6K2 deficiency alleviated DSS-induced colitis symptoms—such as weight loss, colon shortening, and barrier damage—and significantly reduced tumor burden in inflammation-driven carcinogenesis models.
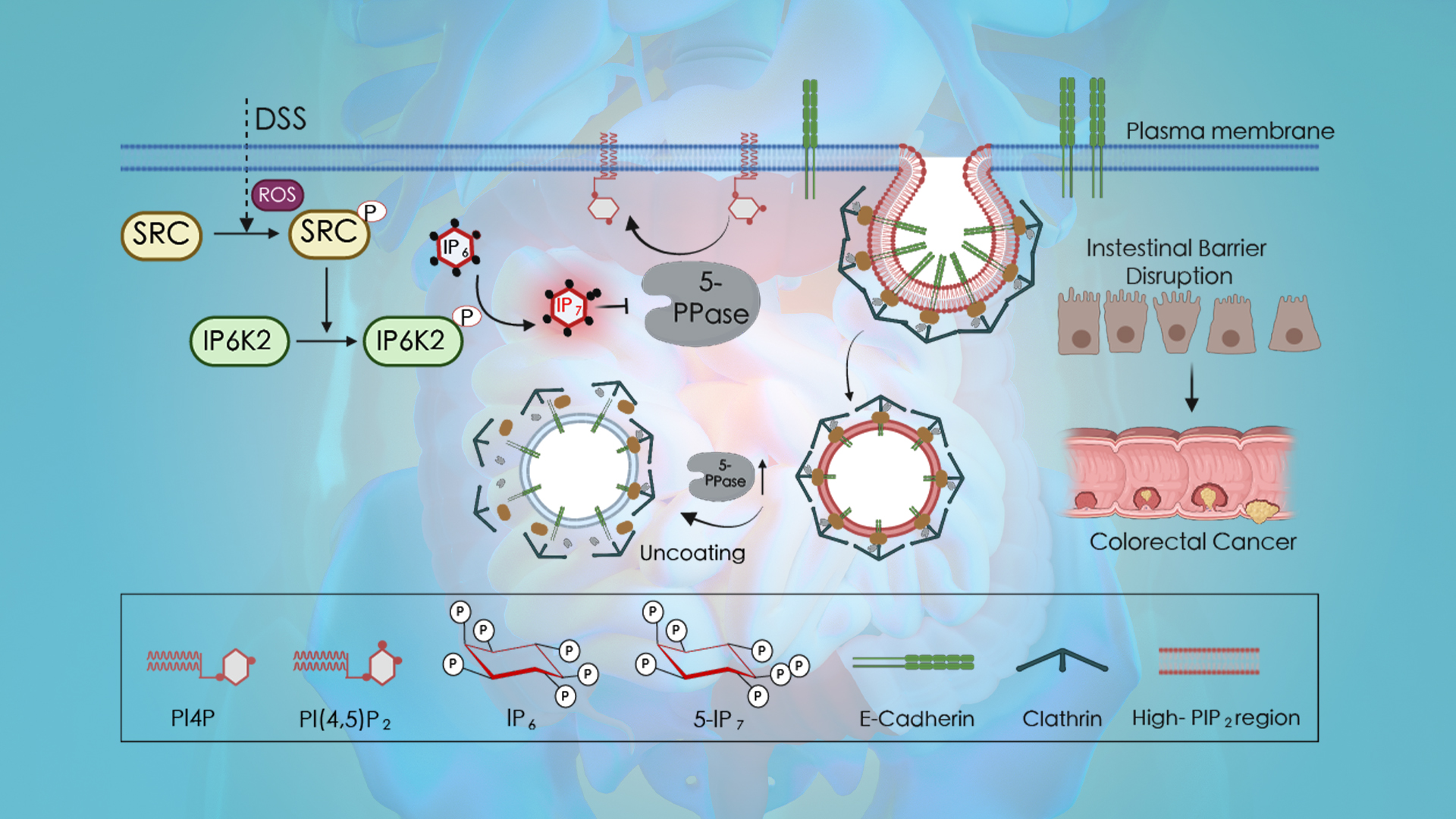
The team revealed for the first time that IP6K2-generated 5-IP7 drives E-cadherin endocytosis, disrupting the epithelial barrier and accelerating inflammation-to-cancer progression. Molecular dissection showed that DSS activates Src kinase via ROS to phosphorylate IP6K2, enhancing its catalytic activity and recruiting it to E-cadherin complexes for localized 5-IP7 synthesis. The 5-IP7 molecule binds to the phosphatase OCRL and competitively inhibits PI(4,5)P2 hydrolysis, triggering PI(4,5)P2 accumulation that drives E-cadherin endocytosis. This cascade disrupts intercellular junctions, liberating β-catenin to activate pro-oncogenic transcription. The study pioneers the novel concept of a “PI(4,5)P2 hydrolysis endocytic checkpoint,” elucidating how inflammatory signals hijack endocytic machinery via 5-IP7 to propel carcinogenesis. Consequently, the screened IP6K2 inhibitor isorhamnetin (ISO) effectively suppressed colitis and tumor progression in murine models.
These findings demonstrate that targeting the IP6K2-5-IP7 axis enables a paradigm shift from reactive treatment to proactive interception of inflammation-driven malignancy before neoplastic transformation, providing innovative therapeutic strategies for barrier-disruption pathologies.
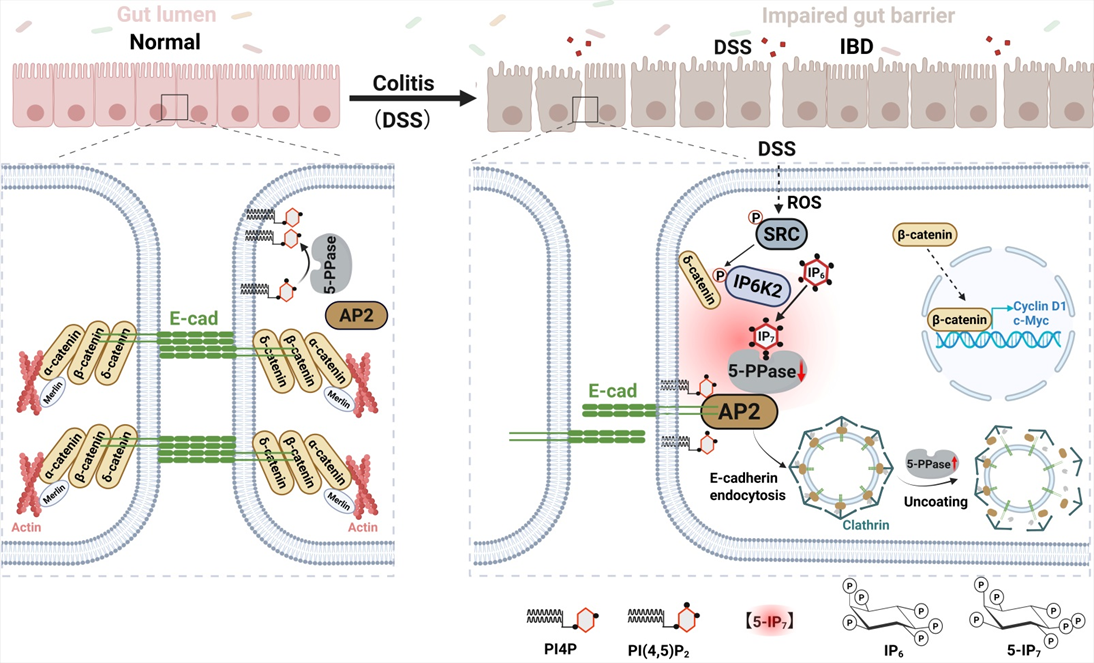
Figure 1. Scheme depicting the role of 5-IP7 in intestinal epithelial barrier (IEB) disruption and oncogenic progression
DSS-induced, Src-mediated tyrosine phosphorylation promotes IP6K2 binding to catenins while stimulating its catalytic activity. 5-IP7 then locally inhibits 5-phosphatases such as OCRL, to promote PI(4,5)P2-mediated recruitment of AP2 and clathrin to displace catenins for E-cadherin endocytosis. The consequent breach of IEB initiates colitis development. Meanwhile, E-cadherin endocytosis permits β-catenin nuclear translocation to promote pro-proliferative transcription.
Ph.D. student Hongyun Zhang, postdoctoral researcher Bobo Zhang, master’s student Yuebo Zhao, and Research Assistant Professor Yang Su from Feng Rao’s group are co-first authors of the paper. Other collaborators include Professor Changzheng Du’s team from Beijing Tsinghua Chang Gung Hospital, Tsinghua University; Professor Niu Huang’s team from the National Institute of Biological Sciences, Beijing; Professor Li Zhao’s team from Tianjin Medical University; Professor Eiichiro Nagata’s team from Tokai University; Professor Henning Jessen’s team from the University of Freiburg; and Professor Philip Hawkins’ team from the Babraham Institute.
Paper link: https://www.nature.com/articles/s41589-025-02005-z
Previous research in Nature Communications (2021): https://www.nature.com/articles/s41467-021-22941-3
Previous research in Nature Metabolism (2021): https://www.nature.com/articles/s42255-021-00468-7
Professor Feng Rao’s webpage: https://faculty.sustech.edu.cn/raof/en/
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yifei REN
Photo ByYan QIU