Chiral hindered dialkyl ethers are important structural motifs and building blocks frequently found in natural products and bioactive molecules. The catalytic asymmetric synthesis of such chiral structural motifs has long been a major goal in modern asymmetric chemistry and has attracted widespread attention from both academic and industrial communities. Aliphatic alcohols are among the most readily available raw materials in the chemical industry and have significant advantages for the preparation of chiral hindered dialkyl ethers via transition-metal-catalyzed asymmetric O-alkylation.
However, the current developed asymmetric catalytic strategies still fail to achieve the efficient asymmetric synthesis of sterically hindered chiral dialkyl ethers—particularly α,α’-trisubstituted α-chiral ethers. The main challenges arise from several factors: the highly sterically hindered nature of tertiary electrophiles and tertiary aliphatic alcohols, resulting in low reactivity; the difficulty in enantiodiscriminating the three substituents of tertiary electrophiles, leading to poor enantiocontrol; and the weak nucleophilicity of tertiary aliphatic alcohols, resulting in poor chemoselectivity.

A research team led by Chair Professor Xin-Yuan Liu, in collaboration with the Assistant Professor Peiyuan Yu’s team, both from the Department of Chemistry at the Southern University of Science and Technology (SUSTech), has made significant progress in the field of asymmetric O-alkylation of aliphatic alcohols for the synthesis of sterically hindered chiral dialkyl ethers.
Their work, titled “Copper-catalysed enantioconvergent O-alkylation of alcohols with racemic α-tertiary haloamides to access enantioenriched hindered dialkyl ethers,” has been published in the international journal Nature Catalysis.
Xin-Yuan Liu’s team has long been dedicated to the study of radical asymmetric catalysis. In recent years, they have developed a copper-catalyzed asymmetric radical cross-coupling strategy. By ingeniously utilizing an out-of-sphere nucleophilic attack mechanism, this approach provides a new method for nucleophiles that are difficult to metallate to participate in catalytic radical asymmetric cross-coupling reactions (Nature 2023, 618, 294).
Based on this strategy, the researchers designed a novel amide-derived anionic N,N,N-tridentate chiral ligand system that combines a sterically unencumbered chiral coordination center with a remote branched side arm. When coordinated with copper, this ligand forms a saturated Cu(III) intermediate. This structure not only suppresses side reactions such as β-hydride elimination but also provides substantial space around the metal center, allowing it to accommodate highly sterically hindered tertiary alcohols, thereby enhancing both chemoselectivity and reaction efficiency.
The introduction of a side arm remote from the metal center in the chiral catalyst could increase the difference in steric repulsion from substituents at the chiral center, leading to significantly improved enantiocontrol. The reaction demonstrates a broad substrate scope, with over 110 examples including primary, secondary, and tertiary aliphatic alcohols bearing diverse functional groups—from commodity chemicals such as methanol and ethanol, to complex bioactive and pharmaceutical molecules. This method allows rapid construction of high-value-added chiral sterically hindered dialkyl ethers.
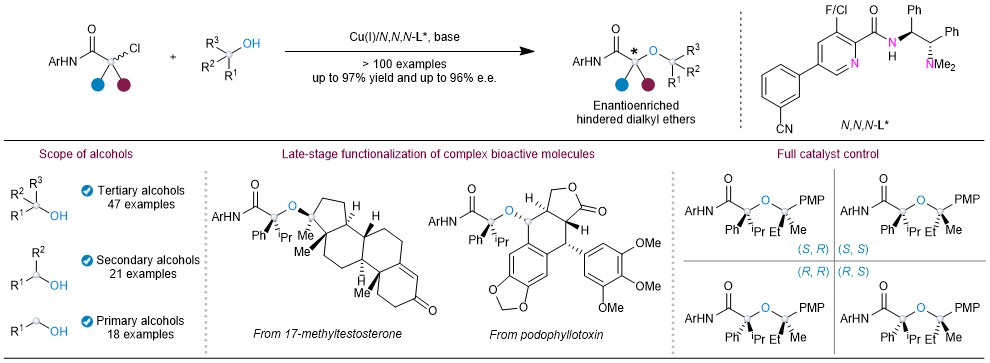
Figure 1. Copper-catalysed enantioconvergent O-alkylation of alcohols with racemic α-tertiary haloamides
This study presents a general strategy for copper-catalysed enantioconvergent radical O-alkylation of diverse alcohols with racemic α-tertiary haloamides. The key to this success lies in the development of anionic N,N,N-ligands with a rigid side arm that create coordinatively saturated Cu(III) intermediates with low steric congestion around the metal center. This method establishes a flexible platform for the construction of structurally diverse and synthetically challenging enantioenriched hindered ethers, especially α,α’-trisubstituted α-stereogenic ones that have not been previously disclosed.
Importantly, the method enables a catalytic stereodivergent process, allowing access to all possible stereoisomers of hindered dialkyl ethers with two stereocenters with high catalyst-controlled stereoselectivity. This strategy will inspire more efforts in the development of asymmetric alkylation reactions of sterically congested nucleophiles derived from readily available starting materials, ultimately benefitting the synthetic community and related research areas.
Distinguished Research Fellow Jia-Yong Zhang from Great Bay University, together with Research Assistant Professor Ji-Jun Chen and Dr. Boming Shen from the Department of Chemistry at SUSTech, are the co-first authors of this paper. Chair Professor Xin-Yuan Liu and Assistant Professor Peiyuan Yu are the co-corresponding authors, and SUSTech is the primary and corresponding affiliated institution.
Paper link: https://doi.org/10.1038/s41929-025-01402-w
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yifei REN
Photo ByYan QIU