Recently, Professor Fuzeng Ren’s research group from the Department of Materials Science and Engineering at the Southern University of Science and Technology (SUSTech) has made significant advances in the field of Orthopedic Biomaterials. These results were published in Advanced Functional Materials, Advanced Healthcare Material, Acta Biomaterialia, Additive Manufacturing, and Journal of Materials Science & Technology.
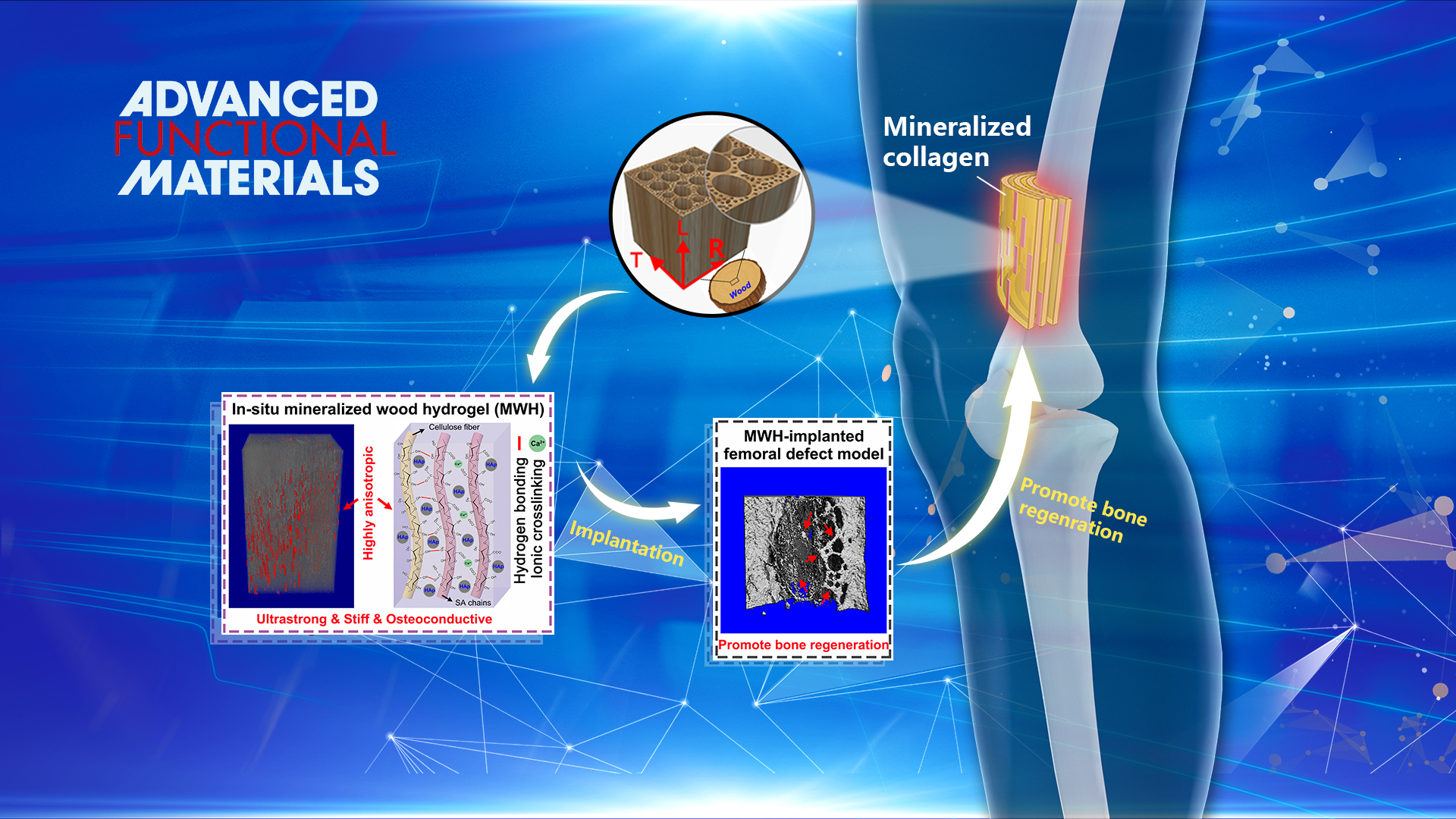
Design and fabrication of artificial tissue engineering scaffold with outstanding mechanical performance and multiple biological functions by mimicking the bone structure is challenging. Natural bone shows a multi-scale hierarchical anisotropic structure consisting of highly ordered staggered arrays of collagen fibrils embedded with plate-like Hydroxyapatite (HAp) nanocrystals. This precise, fine structure formed by the organic/inorganic building blocks offers natural bone a unique combination of exceptional mechanical properties and biological functions.
Similar to the bone structure, natural wood also presents a hierarchical anisotropic structure at the micro and nano-scales. Inspired by natural bone and wood, Dr. Ren’s group proposed a biomimetic strategy to fabricate highly anisotropic, ultrastrong and stiff, and osteoconductive hydrogel composites via impregnation of biocompatible hydrogels into the delignified wood followed by in situ mineralization of hydroxyapatite (HAp) nanocrystals. The well‐aligned cellulose nanofibrils endow the composites with highly anisotropic structural and mechanical properties.
The strong intermolecular bonds of the aligned cellulose fibrils and hydrogel/wood interaction, and the reinforcing nanofillers of HAp enable the composites remarkable tensile strength of 67.8 MPa and elastic modulus of 670 MPa, three orders of magnitude higher than those of conventional alginate hydrogels. More importantly, the biocompatible hydrogel together with aligned HAp nanocrystals could effectively promote osteogenic differentiation in vitro and induce bone formation in vivo. The bone ingrowth into the hydrogel composite scaffold also yields good osteointegration. This study provides a low‐cost, eco‐friendly, feasible, and scalable approach for fabricating anisotropic, strong, stiff, hydrophilic, and osteoconductive hydrogel composites for bone repair.
This study, entitled “Bioinspired Highly Anisotropic, Ultrastrong and Stiff, and Osteoconductive Mineralized Wood Hydrogel Composites for Bone Repair,” has been published on Advanced Functional Materials. Dr. Xiaofei Wang and Dr. Ju Fang are the first authors of the paper.
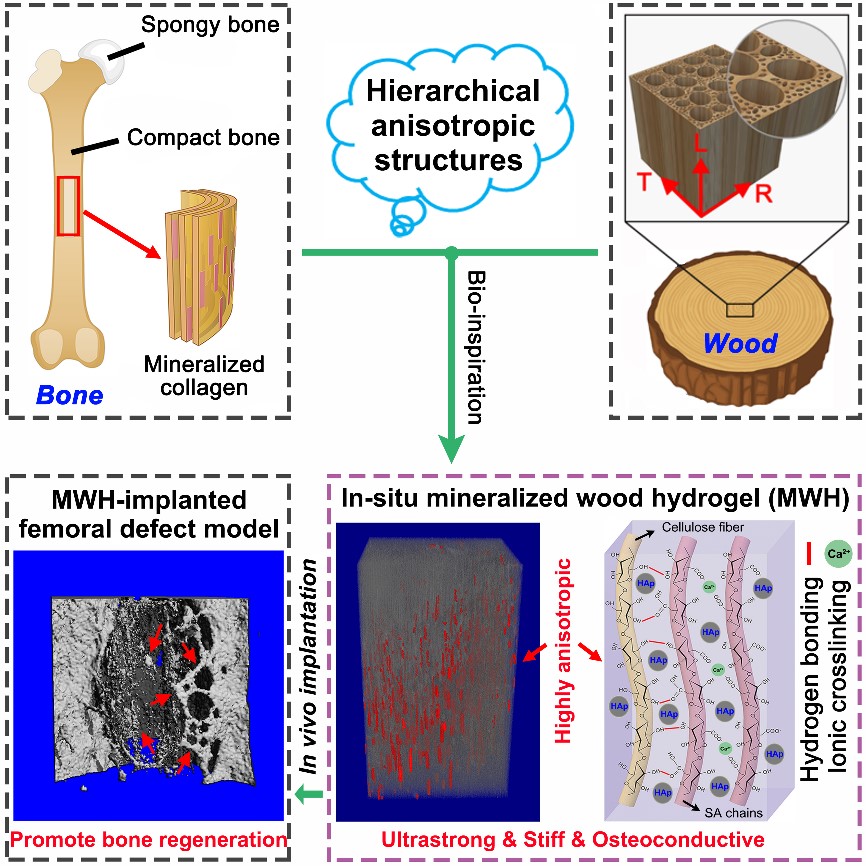
Figure 1. Design strategy, microscopic structure, and in vivo bone formation of the mineralized wood hydrogel composite
Poor osteogenesis and implant‐associated infection are the two leading causes of failure for dental and orthopedic implants. Surface design with enhanced osteogenesis often fails in antibacterial activity or vice versa. To solve this issue, Dr. Ren’s group recently developed a surface design strategy that overcomes this trade‐off via the synergistic effects of topographical micropatterning and a bilayered nanostructured metallic thin film.
A specific micro-grooved pattern is fabricated on the titanium surface, followed by sequential deposition of a nanostructured copper (Cu)‐containing tantalum (Ta) (TaCu) layer and a pure Ta cap layer. The micro-grooved patterns coupled with the nano-rough Ta cap layer shows strong contact guidance to preosteoblasts and significantly enhances the osteogenic differentiation in vitro, while the controlled local sustained release of Cu ions is responsible for high antibacterial activity.
Importantly, rat calvarial defect models in vivo further confirm that the synergy of micro-grooved patterns and the Ta|TaCu bilayered thin film on titanium surface could effectively promote bone regeneration. The present effective and versatile surface design strategy provides significant insight into intelligent surface engineering that can control biological response at the site of healing in dental and orthopedic implants.
These results were published in Advanced Healthcare Materials, entitled “The synergy of topographical micropatterning and Ta|TaCu bilayered thin film on titanium implants enables dual-functions of enhanced osteogenesis and anti-infection.” Mr. Mingyu Zhu, a Ph.D. student of the Department of Materials Science and Engineering at SUSTech is the first author of this paper.
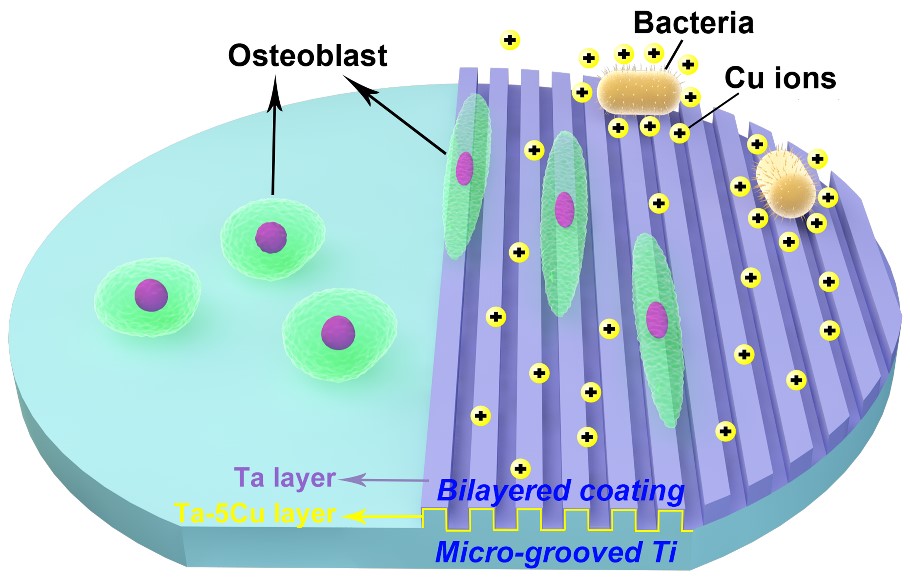
Figure 2. Schematical illustration of the design strategy of the Ta|TaCu bilayered film on medical titanium surface with the anti-bacterial mechanism
Three-dimensional (3D) porous zinc (Zn) with a moderate degradation rate is a promising candidate for biodegradable bone scaffolds. However, the fabrication of such scaffolds with adequate mechanical properties remains a challenge. Moreover, the composition, crystallography, and microstructure of the in vivo degradation products formed at or near the implant-bone interface are still not precisely known.
Based on these considerations, Dr. Ren’s group has fabricated porous Fe@Zn scaffolds with skeletons consisting of an inner core layer of Fe and an outer shell layer of Zn using template-assisted electrodeposition technique. They systematically evaluated their porous structure, mechanical properties, degradation mechanism, antibacterial ability, in vitro and in vivo biocompatibility. In situ site-specific focused ion beam micromilling and transmission electron microscopy were used to identify the in vivo degradation products at the nanometer scale. The 3D porous Fe@Zn scaffolds show a similar structure and comparable mechanical properties to human cancellous bone. The degradation rates can be adjusted by varying the layer thickness of Zn and Fe.
The antibacterial rates reach over 95% against S. aureus and almost 100% against E. coli. A threshold of released Zn ion concentration (~ 0.3 mM) was found to determine the in vitro biocompatibility. Intense new bone formation and ingrowth were observed despite a slight inflammatory response. The in vivo degradation products were identified to be equiaxed nanocrystalline zinc oxide with dispersed zinc carbonate. This study not only demonstrates the feasibility of porous Fe@Zn for biodegradable bone implants but also provides significant insight into the degradation mechanism of porous Zn in a physiological environment.
The study was published in Acta Biomaterialia, entitled “Cancellous bone-like porous Fe@Zn scaffolds with core-shell-structured skeletons for biodegradable bone implants.” Dr. Jin He, Research Assistant Professor of the Department of Materials Science and Engineering at SUSTech, is the first author of this paper.
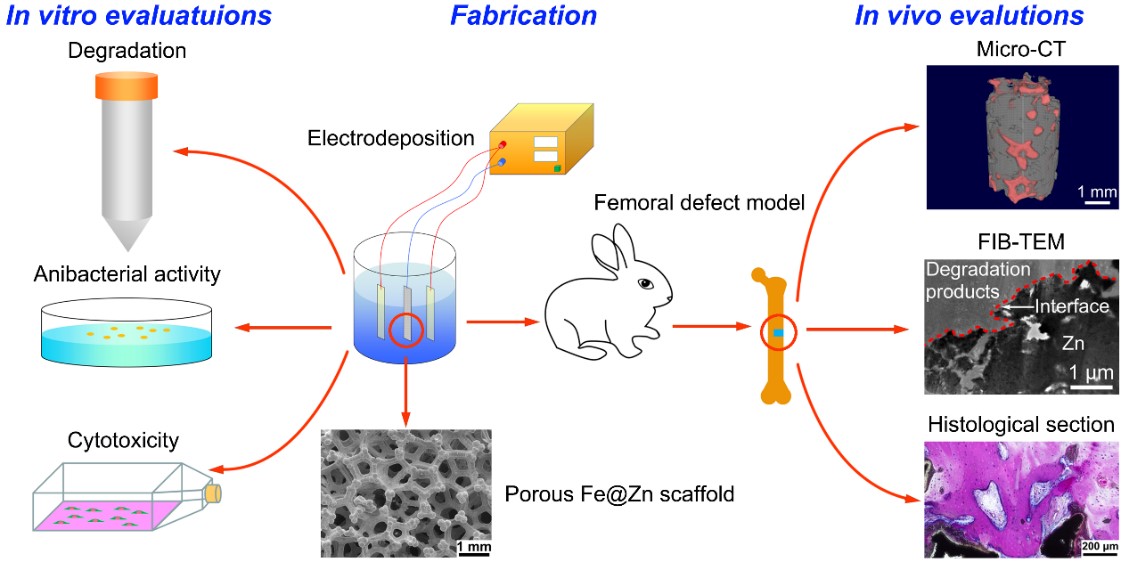
Figure 3. Schematic of fabrication, in vitro, and in vivo evaluations of the Fe@Zn scaffold
Ti-12Mo-6Zr-2Fe alloy, known as TMZF alloy, has been used as a femoral stem in total hip replacement since the early 2000s due to its outstanding biomechanical compatibility and corrosion resistance. Mechanical properties of the alloys used for biomedical implants require not only high strength and ductility but also low elastic modulus to reduce stress shielding. Moreover, the fabrication of TMZF alloy through conventional methods, such as casting, wrought, and forging is costly and time-consuming, while the subsequent machining for the complex-structured biomedical implant brings a big challenge.
In recent years, 3D printing technology has shown its efficiency and cost-effectiveness for customizing complex bone implantations. Recently, Dr. Ren’s group has fabricated a metastable β TMZF alloy with a highly dense structure by 3D printing from low-cost elemental powders. The TMZF alloy demonstrates comparable strength to the Ti-6Al-4V alloy but a much lower elastic modulus, suggesting that it could be a desirable candidate for some implant applications. This work not only provides significant insights into the fabrication of biocompatible β Ti alloy via in-situ alloying-based 3D printing, but also advances the mechanistic understanding of the relationship between processing strategies, microstructural characteristics, and tensile properties of additively manufactured Ti alloy.
This study, entitled “A high strength and low modulus metastable β Ti-12Mo-6Zr-2Fe alloy fabricated by laser powder bed fusion in-situ alloying,” was published in Additive Manufacturing. Ranxi Duan, a Ph.D. student of SUSTech and the University of Birmingham, is the first author of this paper.
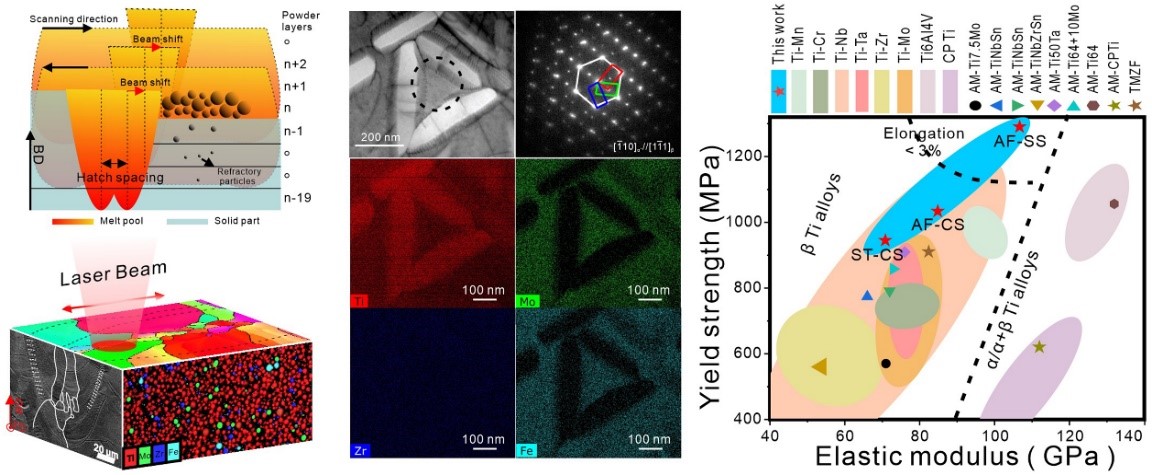
Figure 4. Preparation of the metastable β TMZF alloy by laser powder bed fusion in-situ alloying, the microstructure of the presented TMZF alloy, and the comparative study of its mechanical properties with other biomedical Ti alloys
Refractory metal niobium (Nb) is a potential orthopedic implant material, which has shown notable anti-corrosion, anti-wear, high ductility, low magnetic susceptibility, and good biocompatibility both in vivo and in vitro. However, traditional metal Nb with a coarse-grained structure has some drawbacks such as low strength and lack of antibacterial activity, which has limited its wide application in orthopedic implant materials. Dr. Ren’s group has recently fabricated the nanostructured Nb-5 at.% Ag alloy by mechanical alloying and spark plasma sintering. Compared with pure Nb, Nb-5 at.% Ag alloy has remarkably enhanced comprehensive strength (1486 MPa) and fracture strain (35%).
The second phase, Ag particles, precipitated from the Nb matrix not only reduced friction and wear for the alloy, but also empowers Nb the high antibacterial activity (rate up to 99%). The in vivo implantation results also demonstrate its excellent biocompatibility, anti-infection, and osseointegration ability. This Nb-based nanostructured alloy with excellent mechanical, anti-corrosion, biocompatibility, and antibacterial properties is a promising candidate for orthopedic implant material.
This study, entitled “A high strength, wear and corrosion-resistant, antibacterial and biocompatible Nb-5 at.% Ag alloy for dental and orthopedic implants,” was published in the Journal of Materials Science & Technology. Research Assistant Mr. Tian Wan and Mr. Kangjie Chu of the Department of Materials Science and Engineering at SUSTech were the first authors of this paper.
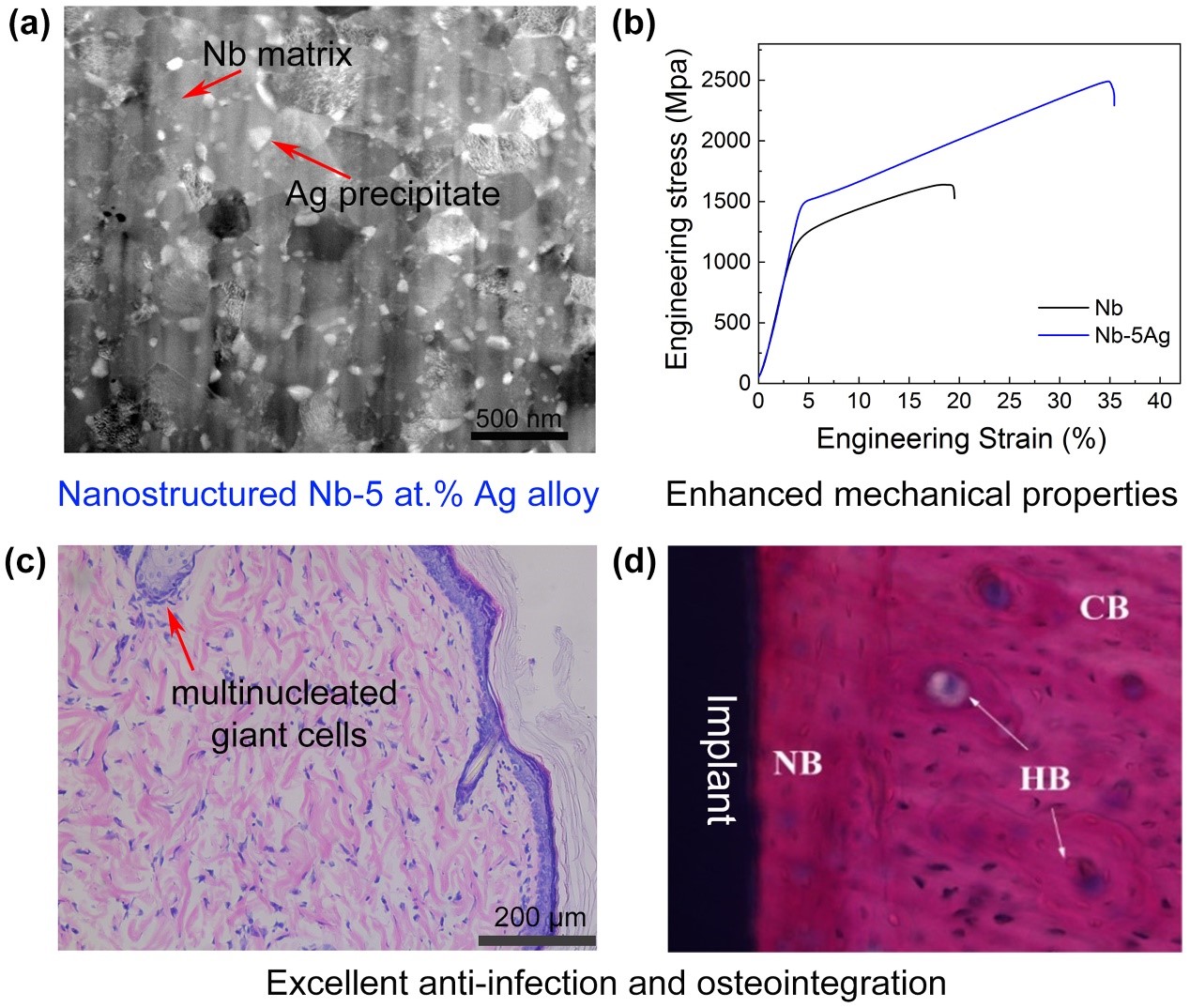
Figure 5. The nanostructure, mechanical strength, anti-infection property, and osteointegration of Nb-5 at.% Ag alloy
Paper links:
https://doi.org/10.1002/adfm.202010068
https://doi.org/10.1002/adhm.202002020
https://doi.org/10.1016/j.actbio.2020.11.032
Proofread ByAdrian Cremin, Yingying XIA
Photo By