Chemical doping is the key process for investigating charge transport in organic semiconductors and improving certain (opto)electronic devices, while n(electron)-doping is fundamentally more challenging than p(hole)-doping. Ideal molecular n-dopant should exhibit high reducing power, good air stability, and high doping efficiency.
A team led by Professor Xugang Guo from the Department of Materials Science and Engineering (MSE) at the Southern University of Science and Technology (SUSTech) has made significant progress in the n-doping of organic semiconductors research. In doing so, they have discovered a highly efficient catalyzed n-doping method.
Their results, entitled “Transition metal catalysed molecular n-doping of organic semiconductors,” have been published in one of the world’s leading multidisciplinary science journals, Nature.
Applying the metal-catalyzed molecular n-doping
As depicted in Figure 1, direct n-dopants reduce an n-type organic semiconductor (acceptor) by direct electron transfer. Therefore, their ionization potential needs to be higher than the electron affinity (EA) of organic semiconductor acceptors, making them sensitive to oxygen/moisture and difficult to handle.
Air-stable molecular n-dopants, which have a deep ionization potential in their initial forms, undergo chemical bond cleavage reactions to generate active-doping-species in situ before electron transfer occurs
However, the doping reaction’s thermodynamics and activation energies strongly affect their reducing strength and reaction kinetics. This results in low doping efficiency and prevents the realization of ideal molecular n-doping technology for organic electronics.
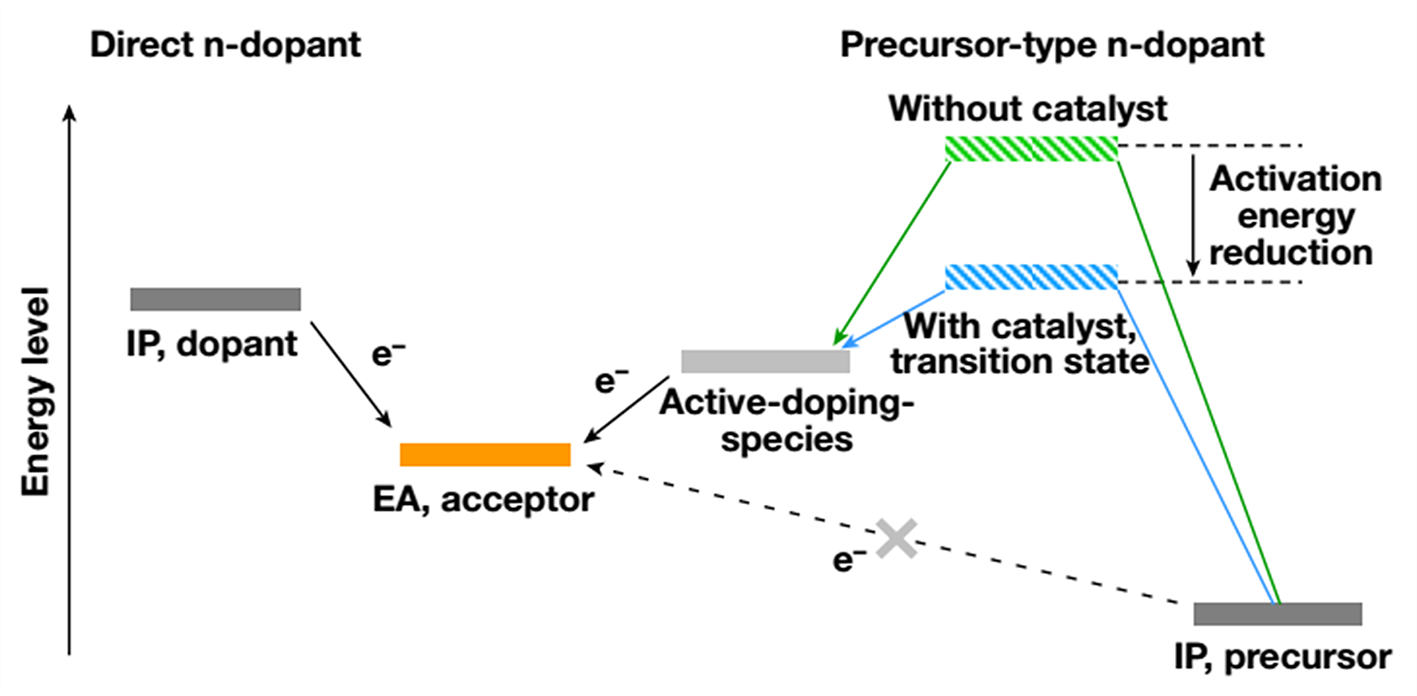
Figure 1. The general concept of transition metal-catalyzed molecular n-doping. Incorporation of transition metal catalyst (blue path) can lower the doping activation energy, increase both the reaction rate and doping efficiency by several orders of magnitude.
The group reports a general method based on transition metal catalysis to tackle this problem in this research. As shown in Figure 1, by introducing transition metal nanoparticles (for example, Pd, Pt, Au) or solution-processable organometallic complex (for example, Pd2(dba)3) as a catalyst to reduce the activation energy to their active-doping-species, it is expected that the reaction rate and extent of doping will greatly increase.
Demonstrating the doping reaction with and without catalyst
The transition metal-catalyzed n-doping technology was demonstrated using a classic molecular n-dopant N-DMBI-H. Without a catalyst, it typically shows low doping efficiency even after a long thermal activation time for several hours. In contrast, incorporation of the Au nanoparticle catalyst can drastically increase its doping efficiency to near 100% in a much shorter doping time of <10 s (Video 1). This enables high electrical conductivity >100 S/cm in solution-processed n-doping polymer semiconductor films.
Video 1. Comparison of the doping reaction without catalyst (left) and with an Au nanoparticle catalyst (right) in ambient air at room temperature. Fast doping reaction with strong solution color change occurs in the presence of an Au nanoparticle catalyst.
A detailed doping mechanism study (Figure 2) showed that the C–H bond cleavage step of N-DMBI-H dopant becomes barrierless and highly exergonic at the presence of an Au nanoparticle catalyst. The activation energy of the H2 gas product generation step is also significantly lowered, which combined to boost the overall doping reaction rate by about one million times.

Figure 2. DFT calculation results on the doping mechanism of N-DMBI-H dopant, showing the Gibbs free energy change both without catalyst (cyan) and with catalyst (orange).
This catalyzed n-doping concept is generally applicable to different n-type organic semiconductors, molecular dopants, and transition metal catalysts. It is demonstrated in organic thermoelectrics, thin-film transistors, and perovskite solar cell devices requiring high charge carrier density and/or efficient electron injection/transport (Figure 3). It offers a broad exploration space of ternary systems comprising catalysts, molecular dopants, and semiconductors, thus opening new opportunities in n-doping research and applications.

Figure 3. Application of transition metal-catalyzed n-doping technology in organic thermoelectrics and organic thin-film transitions for device performance improvement.
Research Assistant Professor Han Guo from Prof. Xugang Guo’s group is the first author of this paper. Prof. Xugang Guo and Antonio Facchetti from Flexterra Corporation are the co-corresponding authors.
Collaborations included Prof. Simone Fabiano from Linköping University (LiU), Prof. Alessandro Motta from the Sapienza University of Rome, and Prof. Han Young Woo from Korea University. The Department of Materials Science and Technology at SUSTech is the first affiliation of this paper.
This work was supported by the National Natural Science Foundation of China (NSFC), the Department of Science and Technology of Guangdong Province, and the Science, Technology and Innovation Commission of Shenzhen Municipality. The SUSTech Core Research Facilities also supported this work.
Paper link: https://www.nature.com/articles/s41586-021-03942-0
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo By