The local coordination environment (LCE) around catalytically active sites plays a vital role in tuning the activity of electrocatalysts made of carbon-supported metal nanoparticles.
To address the problem of structural instability and low activity of oxygen reduction reaction (ORR) catalysts, researchers have proposed an efficient strategy to regulate the LCE and activity of Pd/X&F–C (X=N, P, S, B) to design high-performance catalysts for DEFCs (Figure 1).
Professor Meng Gu from the Department of Materials Science and Engineering at the Southern University of Science and Technology (SUSTech), alongside Prof. Yang Yang from the University of Central Florida (UCF), Prof. Guofeng Wang from the University of Pennsylvania (UPenn), and Prof. Zhenxing Feng from Ohio State University (OSU) have made a series of advances on electrocatalysis. Their achievements have been published in the high-impact journals Nature Energy and Nature Communications.

Their first paper, entitled “Improving Pd–N–C fuel cell electrocatalysts through fluorination-driven rearrangements of local coordination environment,” was published in Nature Energy.
The researchers found that the F atoms in the Pd/N&F–C catalyst push the N atoms towards Pd, efficiently regulating the LCE of Pd by forming Pd–N active sites for catalytic reactions. The Pd–N on the Pd surface not only weakens the CO adsorption but also creates more accessible catalytic sites for the rapid adsorption of O2 during ORR. Furthermore, the N-rich Pd surface promotes C–C bond cleavage and enables complete ethanol oxidation via a 12-electron pathway for EOR.
When used as an ORR catalyst, Pd/N&F–C delivers a high MA of 4.71 A mgPd-1 at 0.9 ViR-free. Meanwhile, as an EOR catalyst, the peak current density for ethanol oxidation of 26.5 A A mgPd-1 was achieved. When tested in the DEFCs, a maximum power density of 0.57 W cm-2 was obtained with long-term stability for more than 5,900 hours, demonstrating the great promise for practical application.
The proposed strategy was examined using other carbon-supported metal catalysts, showing significantly improved activities and stabilities. Hence, this strategy paves a potential way to resolve the corrosion issue of carbon materials used in various energy devices.
Prof. Meng Gu, Prof. Yang Yang, Prof. Guofeng Wang, and Prof. Zhenxing Feng are the corresponding authors of this paper.
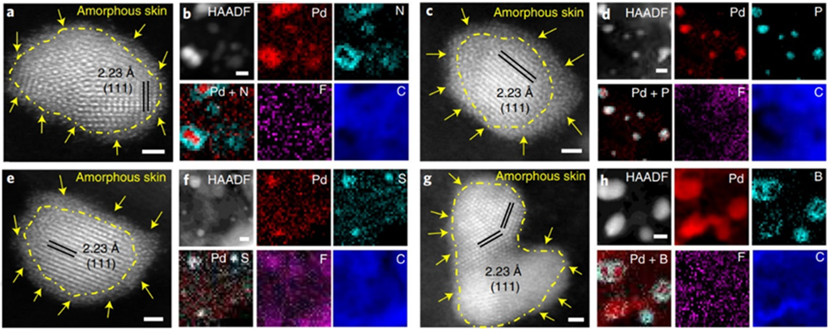
Figure 1. Morphology and atomic structure of Pd/X–F catalysts
The second paper, entitled “A General Strategy for Preparing Pyrrolic-N4 Type Single-Atom Catalysts via Pre-located Isolated Atoms,” was published in Nature Communications.
Prof. Meng Gu, Prof. Jun Li from SUSTech, Prof. Xueliang Sun from the University of Western Ontario, and Prof. Ning Chen from Canadian Light Source Inc. (CLS) have successfully achieved Co1 SAC using Pt1 atoms as catalysts. More importantly, this synthesis strategy can be extended to achieve Fe and Ni SACs (Figure 2).
Single-atom catalysts (SACs) have been applied in many fields due to their superior catalytic performance. Due to the unique properties of the single-atom-site, using the isolated atoms as catalysts to synthesize SACs is promising.
X-ray absorption spectroscopy (XAS) results demonstrate that the achieved Fe, Co, and Ni SACs are in an M1-pyrrolic N4 (M= Fe, Co, and Ni) structure. Density functional theory studies show that the Co(Cp)2 dissociation is enhanced by Pt1 atoms, leading to the formation of Co1 atoms instead of nanoparticles. These SACs are also evaluated under hydrogen evolution reaction (HER) and oxygen evolution reaction, and the nature of active sites under HER are unveiled by the operando XAS studies.
These new findings extend the application fields of SACs to catalytic fabrication methodology, which is promising for the rational design of advanced SACs.
Dr. Junjie Li from the University of Western Ontario, Dr. Qi Wang, and Dr. Yafei Jiang, both from SUSTech, are the co-first authors of this paper. Prof. Meng Gu, Prof. Jun Li, Prof. Ning Chen, and Prof. Xueliang Sun are the corresponding authors.
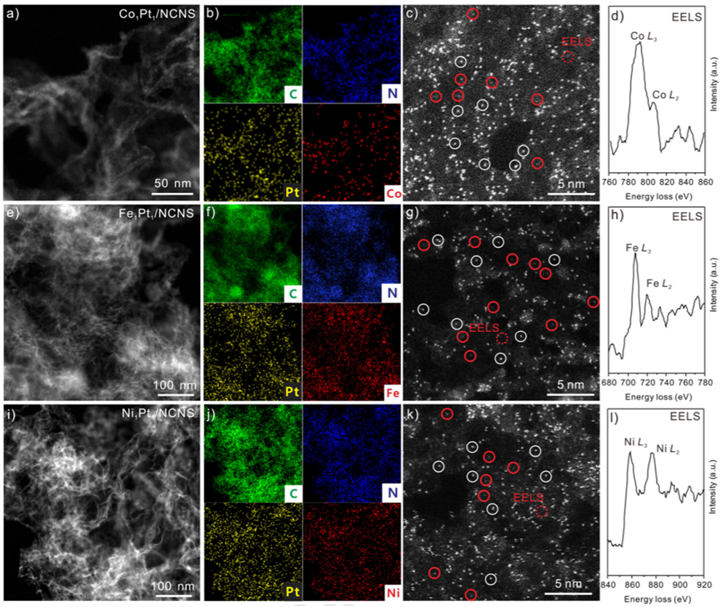
Figure 2. Morphology of M1Pt1/NCNS catalysts
These works were supported by the National Natural Science Foundation of China (NSFC), Entrepreneurial Research Team Program, Shenzhen Peacock Plan, the Shenzhen Clean Energy Research Institute, and the Pico Center at SUSTech.
Paper links:
Nature Energy: https://www.nature.com/articles/s41560-021-00940-4
Nature Communications: https://www.nature.com/articles/s41467-021-27143-5
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo By