Eight-membered ring structural units widely exist in active natural products or drugs. Many natural products with eight-membered rings show very significant physiological activities, and some have been used in clinic settings. For example, the taxol has a unique eight-membered ring. It is one of the best natural anticancer drugs that have been found. It has been widely used in the clinical treatments of breast cancer, ovarian cancer, some head and neck cancer, lung cancer, and others.
The eight-membered ring belongs to the middle ring system and the ring tension is very high. Furthermore, its conformation is highly substrate-dependent. So, the reactivity of the eight-membered ring is difficult to predict. These properties make the synthesis of natural products containing eight-membered rings a formidable challenge.
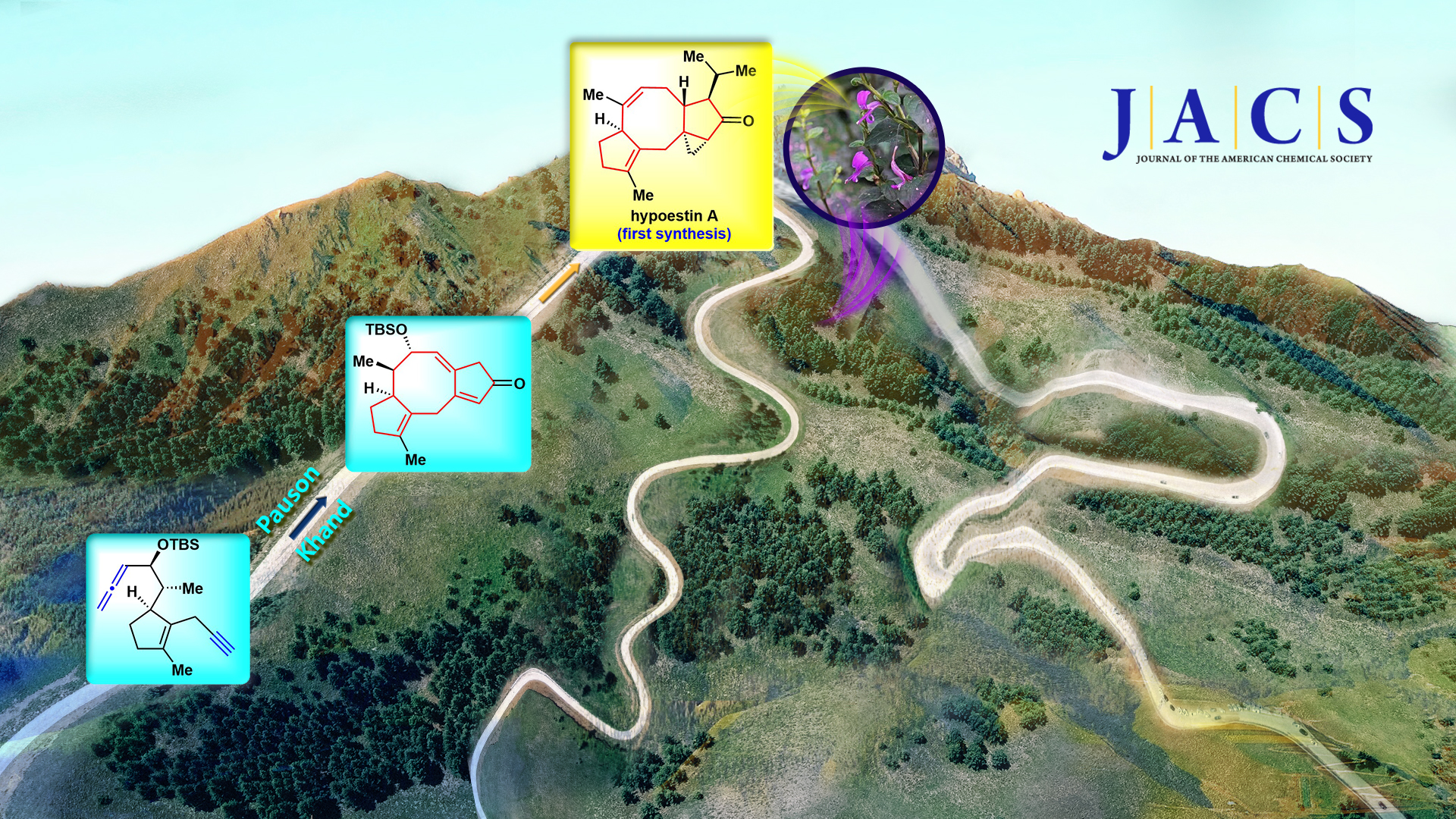
Professor Chuang-Chuang Li’s group from the Department of Chemistry at the Southern University of Science and Technology (SUSTech) has recently developed a new strategy to achieve the high-efficiency total synthesis of three challenging natural products containing eight-membered rings, of which hypoestin A is the first total synthesis.
The study, entitled “Asymmetric total syntheses of hypoestin A, albolic acid, and ceroplastol II,” was published in the Journal of the American Chemical Society (JACS), a high-impact journal that is considered one of the most impactful and prestigious journals in chemistry.
Prof. Chuang-Chuang Li’s group has been committed to the total synthesis of natural products containing eight-membered rings (J. Am. Chem. Soc., 2019, 141, 15773; Chem. Rev., 2020, 120, 5910). In 2021, they developed a concise new asymmetric synthesis strategy and realized the efficient total synthesis of taxol, one of the most difficult molecules in the history of synthesis, with the shortest synthesis route in the world so far (J. Am. Chem. Soc., 2021, 143, 17862).

Figure 1. Natural anticancer drug taxol containing eight-membered ring (highlighted in red).
After continuous efforts and attempts, Prof. Li’s team developed the rhodium-catalyzed intramolecular Pauson-Khand reaction, constructed the challenging [X-8-5] tricyclic system in one step, and applied it to the efficient asymmetric total synthesis of three natural products containing eight-membered rings for the first time. It is worth mentioning that this new strategy has realized the first total synthesis of natural product hypoestin A, and shortened the total synthesis steps of the other two natural products albolic acid and ceroplastol II from 44 steps to 21 steps. In addition, this new strategy will be applied to the total synthesis of more than 400 other natural products with [X-8-5] tricyclic skeleton, which is conducive to their biological activity research.

Figure 2. A) Total syntheses of hypoestin A, albolic acid, and ceroplastol II. B) Other natural products with [X-8-5] tricyclic skeleton.
Yong-Qiang Wang, a doctoral candidate of the Department of Chemistry at SUSTech, is the first author of the paper. Prof. Chuang-Chuang Li is the sole corresponding author, and SUSTech is the sole communication unit. In addition, visiting scholar Kunhua Xu and Research Assistant Professor Long Min from the Department of Chemistry at SUSTech also made important contributions to this paper.
This research was supported by the National Natural Science Foundation of China (NSFC), Guangdong Provincial Key Laboratory of Catalysis, Science and Technology Key Project of Guangdong Province, Research Projects of Universities of Guangdong Province, Graduate Education Innovation Program of Guangdong Province, and the Shenzhen Science and Technology Innovation Commission.
Paper link: https://pubs.acs.org/doi/10.1021/jacs.2c04633
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo By