Li-metal batteries (LMBs) with high-voltage cathodes could achieve extremely high energy densities at the cell level (around or even higher than 350 Wh kg-1). Designing proper electrolytes of high compatibility with Li metal anodes (LMAs) and high voltage resistance is therefore critical for high-energy-density LMBs.
In recent years, high-concentration electrolytes (HCEs) and localized high-concentration electrolytes (LHCEs) have been recently demonstrated, which, however, require an extremely high salt-to-solvent ratio (SSR; SSR ≥ 1 : 2). Decreasing the SSR without sacrificing the electrochemical performance of the LMBs is quite challenging.
Professor Tianshou Zhao’s research group from the Department of Mechanical and Energy Engineering at the Southern University of Science and Technology (SUSTech) has recently reported a strategy for reconstructing the solvent molecules in ether electrolytes, through which the anode stability and high-voltage resistance could be hugely promoted.
Their paper, entitled “A Solvent Molecule Reconstruction Strategy Enabling a High-Voltage Ether-based Electrolyte,” has been published in Energy & Environmental Science, a top journal in the field of energy storage.
The team’s work proposes the strategy of preparing the localized middle concentration electrolyte (SSR = 1:3.6) based on the controllable polymerization of 1,3-dioxolane (DOL). Compared to the unpolymerized liquid electrolyte with the same salt species and concentration (1.3 M LiTFSI + 1.3 M LiFSI-DOL-TTE), the reconstructed electrolyte (Hybrid-DOL/PDOL-TTE) could passivate the LMA and NMC cathode more efficiently, leading to a more stable operation of high-voltage LMBs.
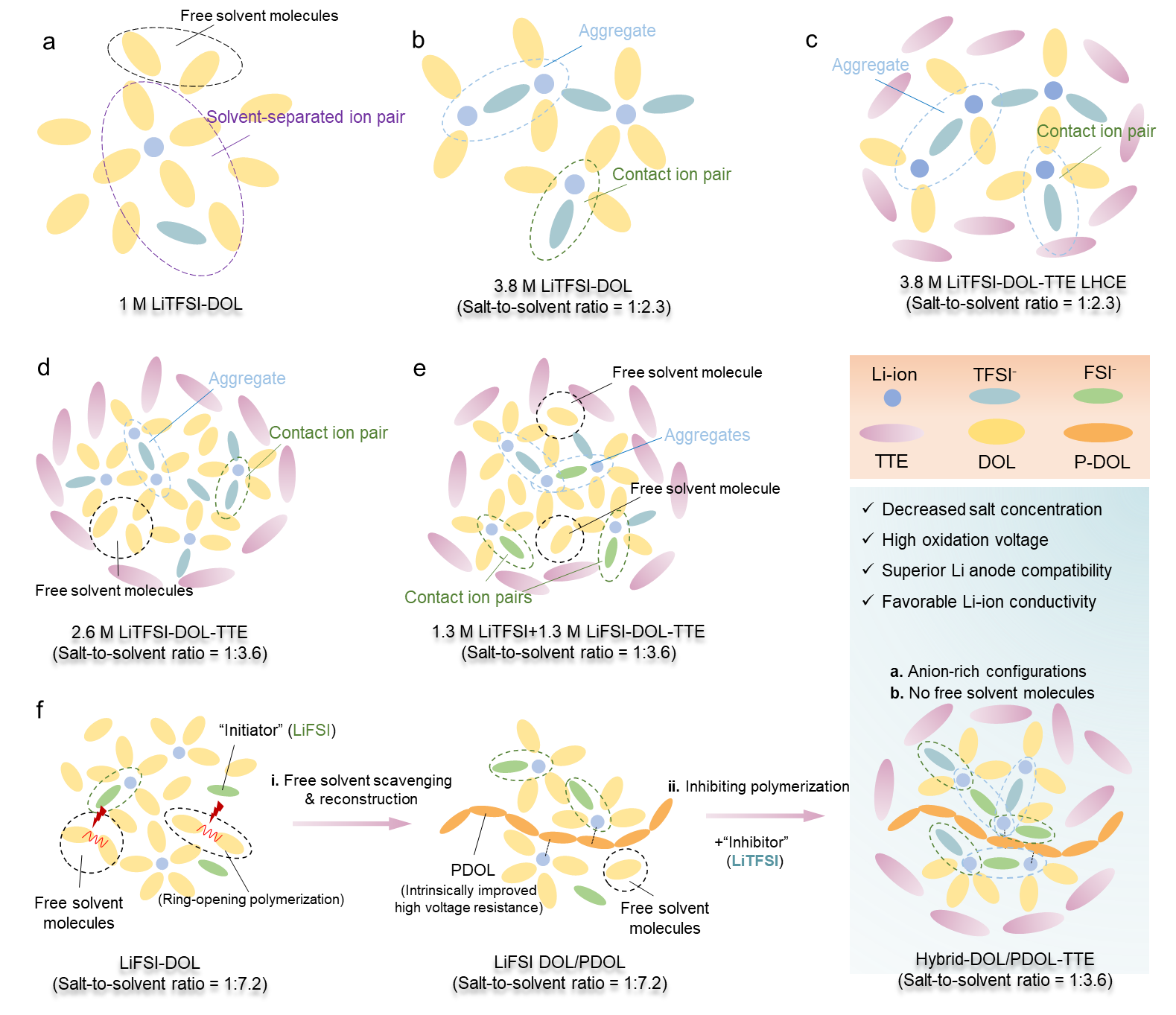
Figure 1. The solvation structures of various electrolyte systems
The results of Raman spectra and MD/DFT calculation show that the partial reconstruction of the DOL molecules through LiFSI could effectively reduce the existence of free/uncoordinated DOL and transform them into intrinsically more stable polymerized DOL (PDOL). Besides, the partial polymerization induces the formation of more anion-rich ion-solvent configurations in the electrolyte.
In the reconstructed electrolyte, Hybrid-DOL/PDOL-TTE, the ion-solvent configurations with anion-to-solvent ratio (ASR) ≥ 2 take up around 19.41%, which is much higher than the unpolymerized 2.6 M LiTFSI-DOL-TTE (7.99 %) and 1.3 M LiTFSI+1.3 M LiFSI-DOL-TTE (12.76 %). The anion-rich configurations have lower LUMOs and HOMOs, and their decomposition would render LiF-rich SEI at the anode and LiF-rich CEI at the cathode, which is able to promote the cyclability of both the LMA and high-voltage cathodes.
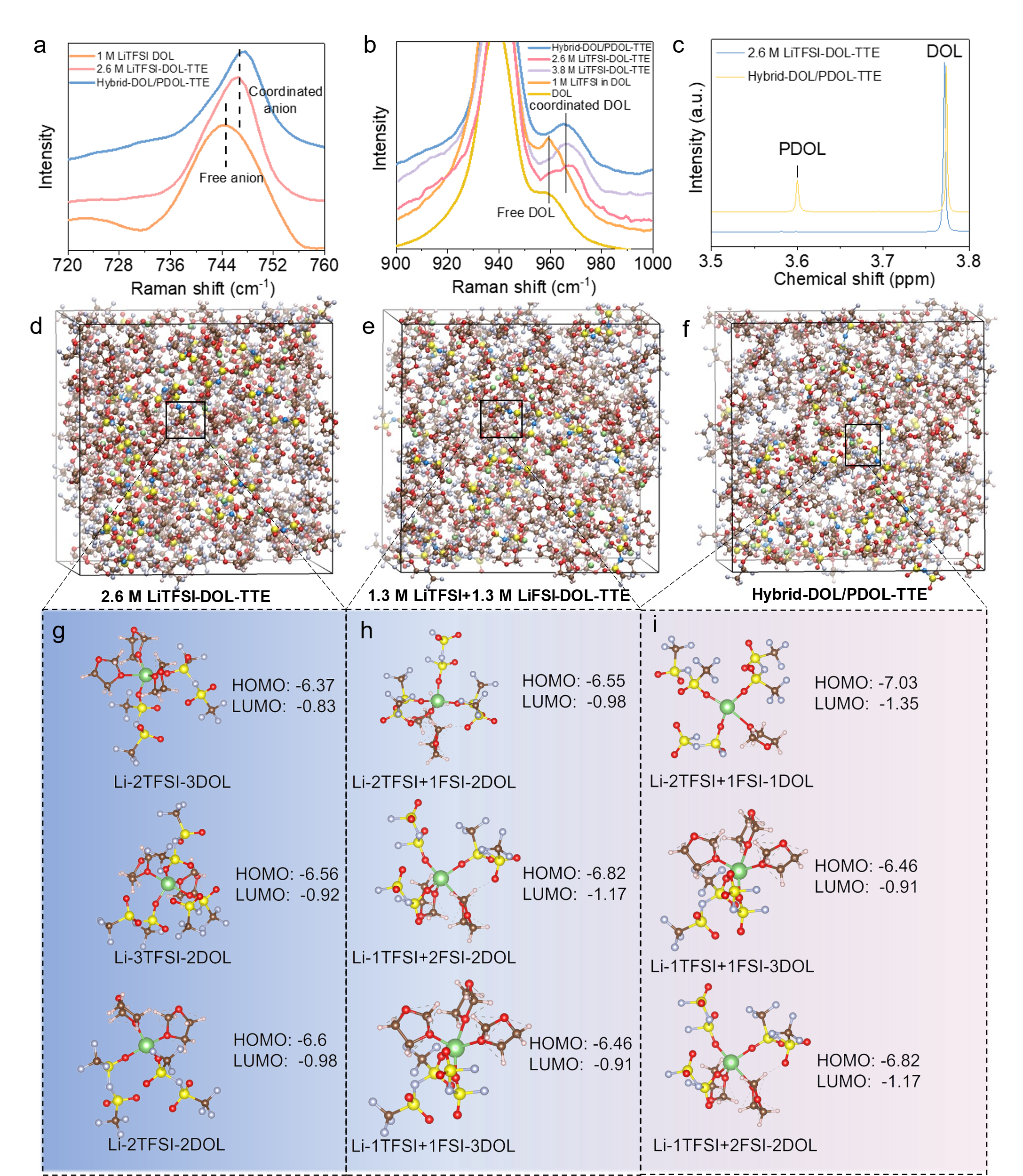
Figure 2. The characterization and MD/DFT calculation of solvation structures of various electrolytes
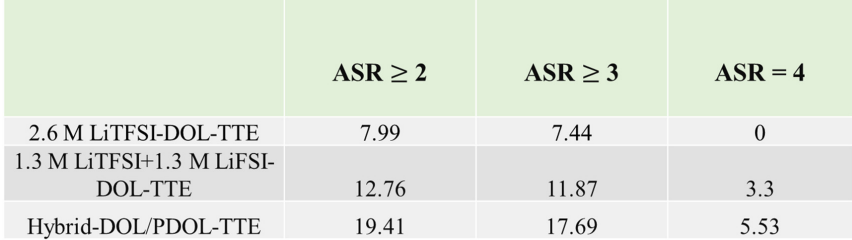
Table 1. The frequencies of configurations with a high anion-to-solvent ratio (≥ 2) in different electrolytes
The researchers test the anode performance based on Li|Cu and Li|Li cell configurations using the reconstructed Hybrid-DOL/PDOL-TTE and the control electrolytes, 2.6 M LiTFSI-DOL-TTE and 1.3 M LiTFSI+1.3 M LiFSI-DOL-TTE. The Li|Cu cell with the Hybrid-DOL/PDOL-TTE electrolyte delivers high Coulombic efficiencies (CEs) up to 99.2% during a long-period cycle. XPS and TOF-SIMS techniques are utilized to investigate the SEI components of LMAs rendered by different electrolytes.
Results from both tests suggest that a lower total amount of organics and higher contents of LiF are derived for the LMA cycled in Hybrid-DOL/PDOL electrolyte. Furthermore, there are abundant polyether-derived segments in the SEI of LMA cycled in Hybrid-DOL/PDOL, which may offer a more elastic and durable network during Li plating/stripping, leading to more stable cycling performances of the anode.
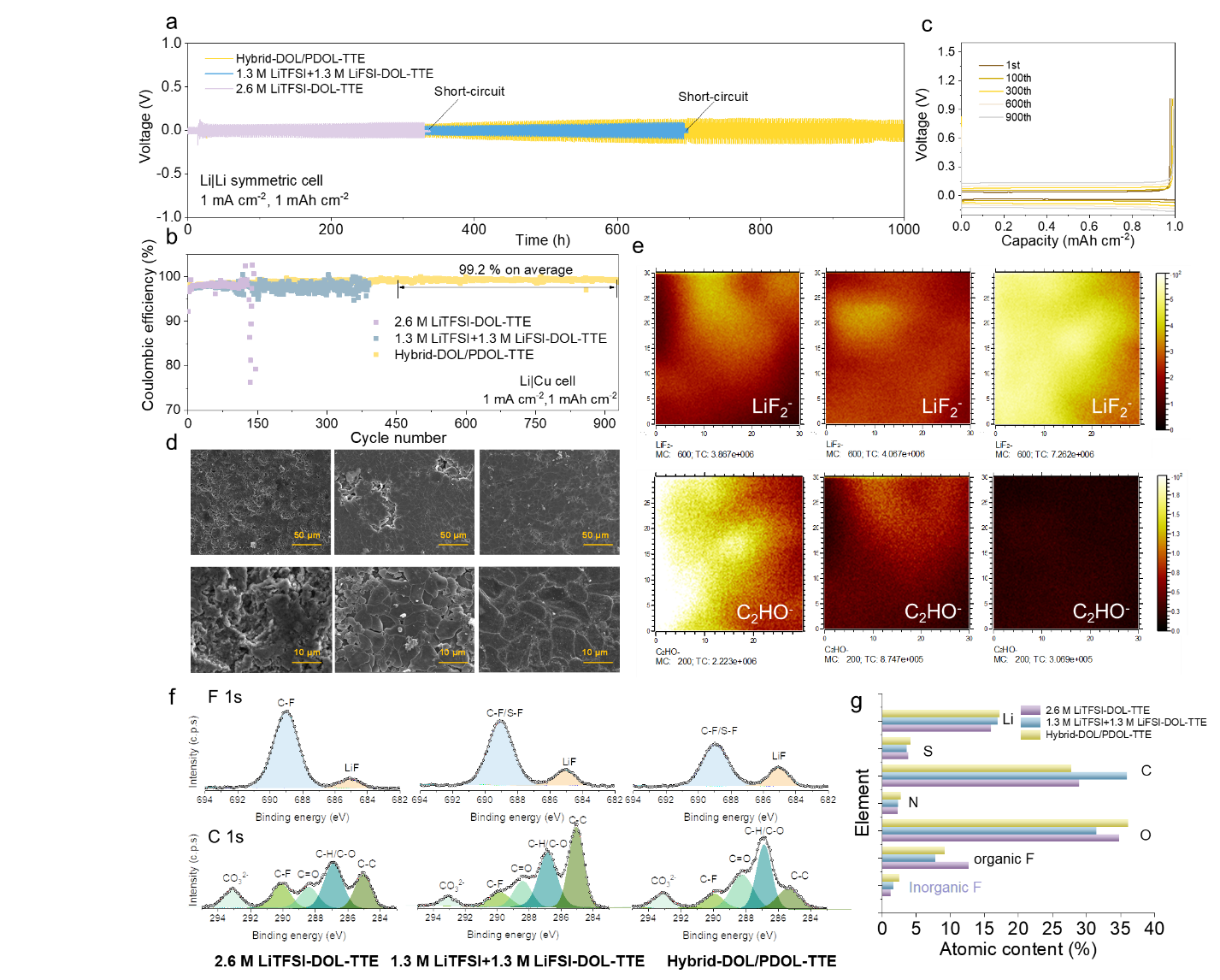
Figure 3. The morphology and component analysis of the cycled LMAs
The promoted performance on the cathode side is even more significant compared to the anode side. The Li|NCM622 cell (2.5-4.4 V) using Hybrid-DOL/PDOL-TTE stably operates for more than 300 cycles with 86% of the initial capacity retained and high CEs (>99.8%) achieved. By contrast, the lifespan of the cell using 1.3 M LiTFSI+1.3 M LiFSI-DOL-TTE comes shorter than 100 cycles). Even at an ultrahigh cut-off voltage of 4.6 V, the cell with reconstructed Hybrid-DOL/PDOL-TTE still shows high capacity retention (>88%) after 100 round cycles, while the cell using 1.3 M LiTFSI+1.3 M LiFSI-DOL-TTE presents accelerated capacity decay and low CEs within a mere 20 cycles.

Figure 4. Electrochemical performance of Li|LFP and Li|NCM622 cells using different electrolytes
Characterizations like FIB-SEM, TEM, and XPS are carried out on the cycled electrodes to explore the origins of improved high-voltage stability. The NCM622 particles cycled in Hybrid-DOL/PDOL-TTE do not show noticeable cracks and gliding planes, unlike that in the 2.6 M LiTFSI-DOL-TTE and 1.3 M LiTFSI+1.3 M LiFSI-DOL-TTE. According to the XPS result, the CEI formed by Hybrid-DOL/PDOL-TTE has evidently higher contents of LiF and polyether-derived segments, and significantly fewer Li2CO3 compared to the other two control electrolytes. The TEM shows that the Hybrid-DOL/PDOL-TTE helps form uniform CEI on the surface of the NCM622 particle. These results indicate Hybrid-DOL/PDOL-TTE could passivate the cathode efficiently by enriching CEI with massive inorganic fluorides and reducing the contents of organics.
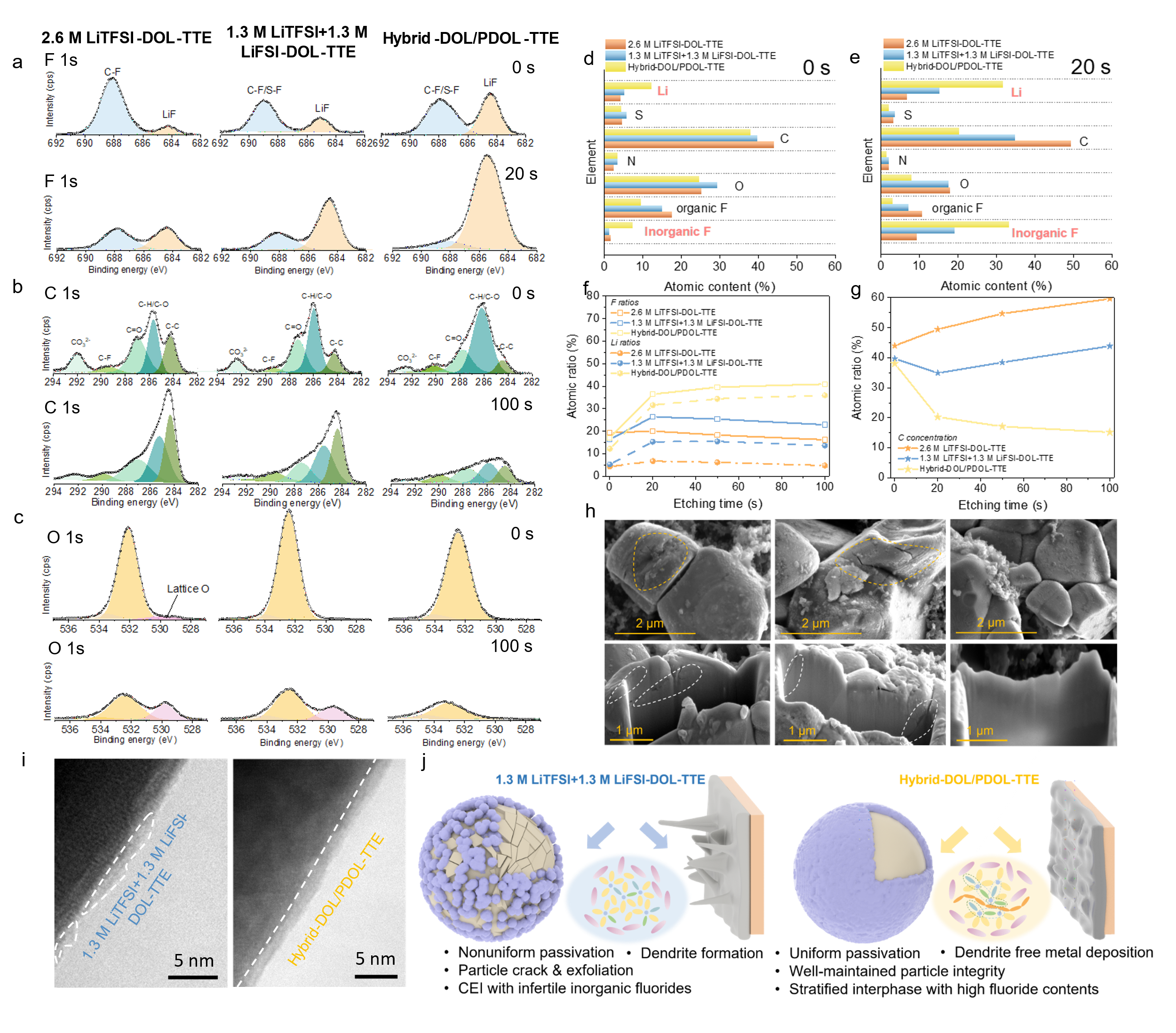
Figure 5. Postmortem analysis of NCM622 particles cycled in different electrolytes
The Li|NCM622 full cells with 20 µm Li foil are tested using the Hybrid-DOL/PDOL-TTE under high cut-off voltages of 4.4 V and 4.6 V, showing stable cycling performance. The pouch cell with the high-loading cathode, limited electrolyte usage, and barren Li (N/P ~ 1.7) enables a high energy density of 347.1 Wh kg-1. These data further evidence the reconstruction strategy essentially upgraded the high-voltage stability of the liquid ether electrolyte.
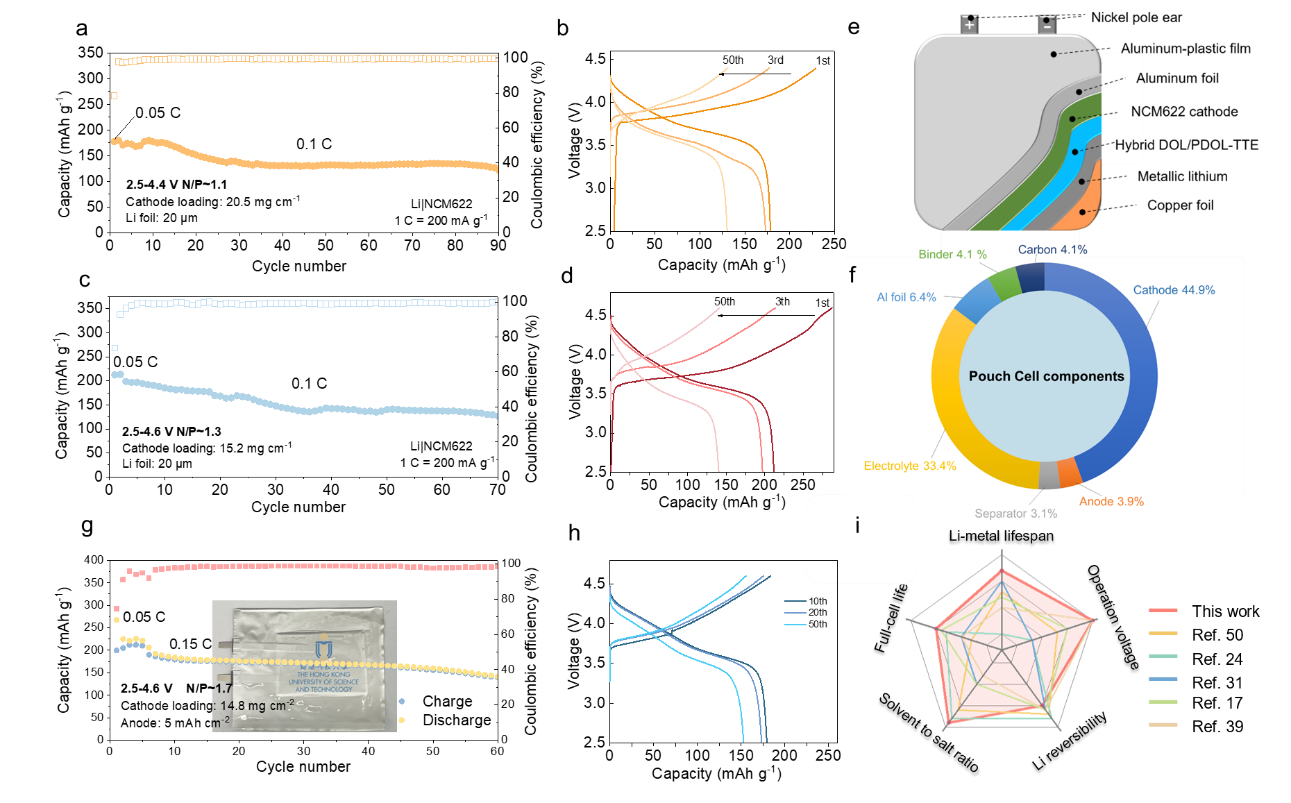
Figure 6. Performances of Li|NCM622 full cells using the Hybrid-DOL/PDOL-TTE electrolyte
In conclusion, the improved anode and cathode performances are enabled by the Hybrid-DOL/PDOL-TTE electrolyte with a low SSR (1:3.6). The scavenging of free DOL molecule, formation of PDOL, and the as-induced more anion-rich configurations promote the intrinsic high-voltage resistance of the ether electrolyte. Meanwhile, the SEI/CEI with rich LiF and polyether-derived segments help stabilize the anode/cathode interfaces.
The Li|NCM622 cells with the reconstructed Hybrid-DOL/PDOL-TTE electrolyte stably operate under high voltages like 4.6 V. The pouch cell with the Hybrid-DOL/PDOL-TTE shows a high specific energy of 347.1 Wh kg-1. This work successfully demonstrated a new solvent molecule reconstruction strategy to prepare a high-performance ether-based electrolyte with a medium salt concentration for high-voltage LMBs.
Mr. Xudong Peng of the Hong Kong University of Science & Technology (HKUST) and Prof. Tianshuai Wang at the Northwestern Polytechnical University are the co-first authors of this paper. Dr. Bin Liu of HKUST is the co-author, while Profs. Tianshou Zhao and Yiju Li at SUSTech are the corresponding authors.
The study was supported by the Research Grants Council of the Hong Kong Special Administrative Region, China.
Paper link: https://pubs.rsc.org/en/content/articlelanding/2022/ee/d2ee02344j
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo By