Sphingolipids are important membrane components and bioactive signaling molecules in all eukaryotic cells, participating in various vital life activities. The level of sphingolipids in organisms needs to be maintained in balance, as too much or too little can have adverse effects on normal growth and responses to the external environment.
In animals, disruption of sphingolipid homeostasis may lead to various diseases, such as cancer, cardiovascular disease, and neurodegenerative diseases. In plants, the stable regulation of sphingolipid levels is crucial for regulating plant growth and responding to various biotic and abiotic stressors. The first step in sphingolipid synthesis in all eukaryotes is catalyzed by the SPT complex on the endoplasmic reticulum membrane, which is also the rate-limiting step of the entire sphingolipid synthesis process. ORM/ORMDL proteins are a key protein family for the stable regulation of SPT activity, and they are conserved in all eukaryotes.

In 2021, Professor Xin Gong’s research team from the School of Life Science at the Southern University of Science and Technology (SUSTech) reported the structure of the human SPT-ORMDL3 complex in its apo and substrate-bound forms, revealing the overall assembly and substrate selectivity of the enzyme’s molecular mechanism (Li et al., Nat. Struct. Mol. Biol., 2021). However, how ORM/ORMDL proteins regulate SPT activity according to cellular sphingolipid levels remains a mystery in all eukaryotes. Based on the previous analysis of the structure and function of the human SPT-ORMDL3 complex, Prof. Gong’s team has further investigated the SPT-ORM1 complex in Arabidopsis.
Their study, entitled “Mechanism of sphingolipid homeostasis revealed by structural analysis of Arabidopsis SPT-ORM1 complex,” was published in Science Advances, uncovering the crucial mechanism of the regulation of sphingolipid synthesis homeostasis in plants.
The researchers first obtained homogenous Arabidopsis thaliana SPT-ORM1 complex protein through recombinant expression and purification. They successfully resolved the three-dimensional structure of the SPT-ORM1 complex using single-particle cryo-electron microscopy technology (Figure 1). Interestingly, they observed noticeable lipid molecule density in the three-dimensional structure, which was subsequently identified as endogenous ceramide molecules through a series of follow-up studies. By mutating the ceramide binding site and conducting corresponding biochemical experiments, they found that ceramide binding played an important role in inhibiting SPT activity. They also demonstrated that ceramide binding could induce and stabilize the N-terminal of ORM1 in a conformation that inhibits substrate binding, thereby reducing SPT activity.
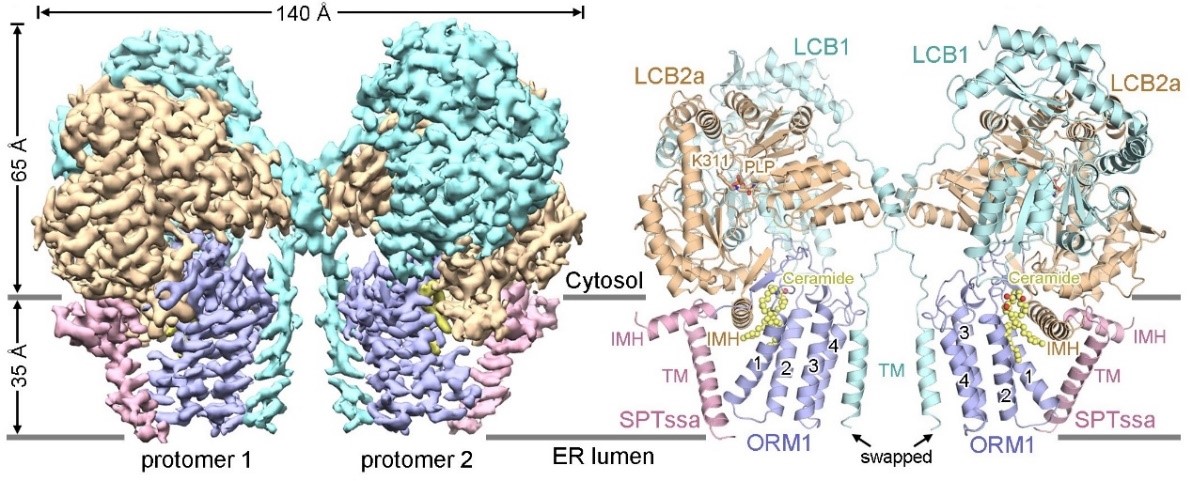
Figure 1. Overall structure of the Arabidopsis SPT-ORM1 complex bound to ceramide
Based on the structural biology findings and extensive biochemical experiments in this study, Prof. Gong’s team finally proposed a working model for the regulation of SPT activity in Arabidopsis (Figure 2). In this model, ORM1 can competitively inhibit SPT through its N-terminus, reducing the affinity of SPT for substrates. More importantly, the SPT-ORM1 complex can serve as a ceramide sensor in the endoplasmic reticulum, controlling the activity of SPT by sensing the levels of ceramide in the ER membrane.
When ceramide levels are low, the highly flexible N-terminal segment of ORM1 cannot effectively inhibit substrate binding, leading to an increase in sphingolipid synthesis to restore sphingolipid homeostasis. As sphingolipid synthesis increases and ceramide accumulates to levels higher than the cell required, ceramide binds to the SPT-ORM1 complex. It induces a hybrid β-sheet between the N-terminus of ORM1 and LCB2a, stabilizing the N-terminal segment of ORM1 in a conformation that inhibits substrate binding and thus downregulating sphingolipid synthesis. Finally, through molecular docking analysis, they found that the mechanism of regulating sphingolipid homeostasis by ceramide-induced regulation of the SPT-ORM1 complex in Arabidopsis may also exist in the human SPT-ORMDL complex, providing an important reference for studying the regulatory mechanisms of sphingolipid synthesis in other higher organisms in the future.
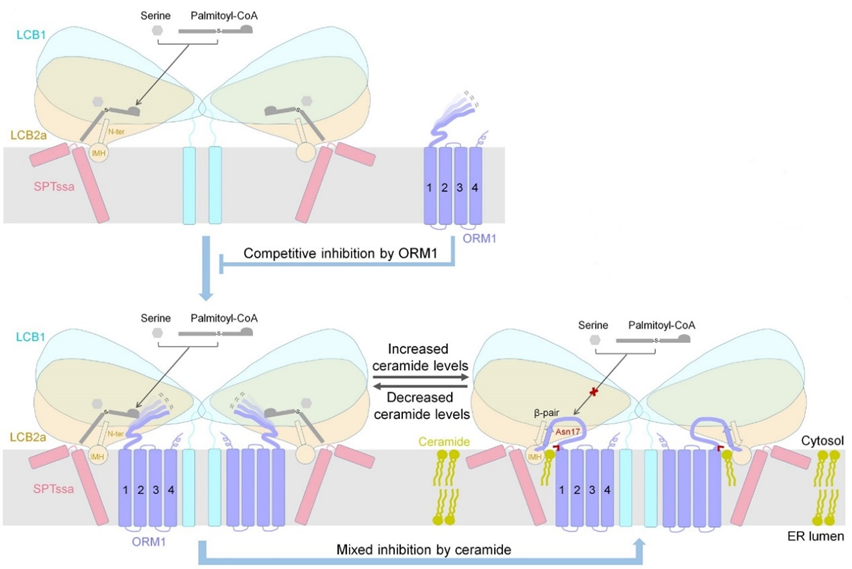
Figure 2. A working model for homeostatic regulation of SPT activity in Arabidopsis
Prof. Xin Gong from the School of Life Science at SUSTech is the corresponding author. Peng Liu and Tian Xie, members of Prof. Gong’s research team, contributed equally to this study. Prof. Teresa M. Dunn from the Unified Service University of Health Sciences, Prof. Edgar B. Cahoon from the University of Nebraska-Lincoln, and Prof. Ruifeng Yao from the Hunan University also contributed to this research.
This research was supported by the National Natural Science Foundation of China (NSFC), Department of Science and Technology of Guangdong Province, Shenzhen Science and Technology Innovation Commission, and the US National Science Foundation. Additionally, the cryo-electron microscopy data collection and processing was supported by the Cryo-Electron Microscopy Center of SUSTech, and the mass spectrometry experiments were supported by the Public Analysis and Testing Center of SUSTech.
Paper link: http://www.science.org/doi/10.1126/sciadv.adg0728
Related link of previous research work published in 2021: https://newshub.sustech.edu.cn/en/html/202102/29991.html
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo By