Recently, a research team led by Dr. Xin Gong from the Department of Biology of Southern Univerity of Science and Technology (SUSTech) published an article, entitled “Structural insights into the assembly and substrate selectivity of human SPT-ORMDL3” in Nature Structural & Molecular Biology. They’ve reported for the first time the three-dimensional structure of human serine palmitoyltransferase (SPT), which is involved in the first step of sphingolipid synthesis and functions as the key enzyme of the rate-limiting step of the entire sphingolipid synthesis process. They’ve also reported the structures of the substrate-free and substrate-bound SPT-ORMDL3 complexes. Together with the biochemical analysis, the study revealed the detailed molecular mechanisms of subunits assembly, substrate binding, and selectivity.
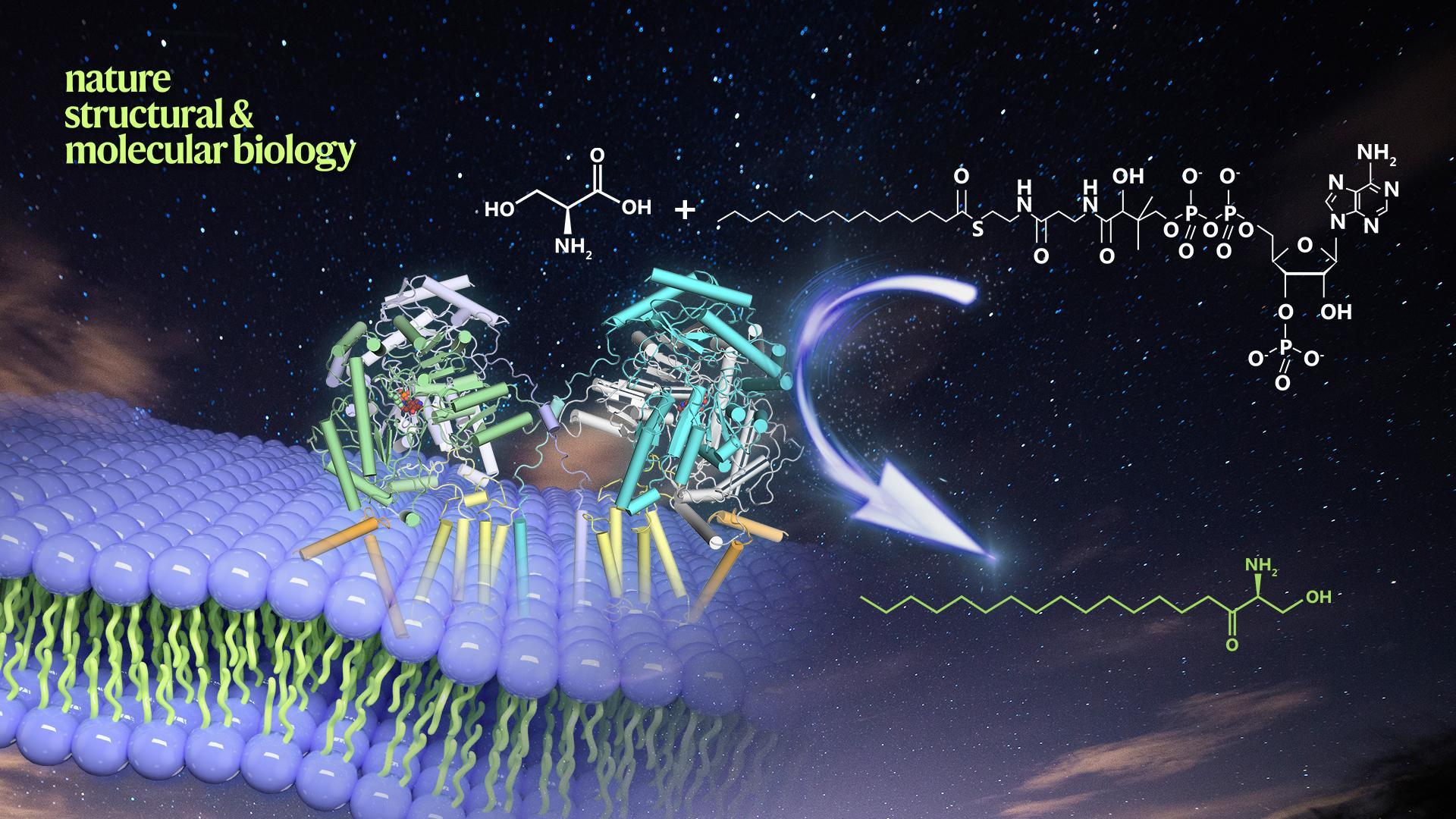
Sphingolipids represent a major class of lipids that serve as not only essential structural components of biological membranes but also critical signaling molecules, and dysregulation of cellular sphingolipid metabolism can lead to various diseases. The synthesis of all sphingolipids in eukaryotes is catalyzed by the SPT heterotrimeric complex on the endoplasmic reticulum membrane. SPT can catalyze the condensation of serine and palmitoyl-CoA to form the sphingolipid precursor 3-ketodihydrosphingosine. Mutations in the human SPTLC1 and SPTLC2 subunits have been shown to cause a slowly progressive neurological disorder known as hereditary sensory and autonomic neuropathy type 1. Basal SPT activity is increased nearly 100-fold in the presence of a small subunit, either SPTssa or SPTssb, and they also determine the acyl-CoA substrate preference, although the precise role of these small subunits remains unknown. Aside from subunit composition, the activity of the SPT complex is also regulated by other proteins. ORMDLs protein is a key regulator of SPT, which can form a stable complex with SPT and participate in regulating the activity of SPT, thereby regulating the homeostasis of sphingolipids in cells. The human ORMDL3 gene is closely related to the susceptibility of childhood asthma. Despite the critical roles of SPT and SPT-ORMDL3 complexes in sphingolipid biosynthesis and pathophysiological processes, structural and in vitro enzymatic characterization of the eukaryotic SPT and its complex with ORMDL3 are currently lacking.
The researchers obtained the human SPT and SPT-ORMDL3 complex by recombinant expression and in vitro purification. And the in vitro catalytic activity of the complex and the corresponding enzyme kinetic parameters were measured for the first time in the study. Then, the researchers used single-particle cryo-EM to determine the high-resolution three-dimensional structures of the human SPT and SPT-ORMDL3 complex (Figure 1). The structure shows that SPTLC1 and SPTLC2 subunits form a dimer of SPTLC1-SPTLC2 heterodimer. The SPTLC1-SPTLC2 heterodimer, together with a SPTssa subunit, form the heterotrimeric catalytic core of SPT. In addition, ORMDL3 is centrally located within the transmembrane region of the complex, thus stabilizing the overall assembly of the SPT complex. In the absence of ORMDL3, the transmembrane region of the SPT complex became more flexible. However, the molecular mechanism of how ORMDL3 regulates SPT activity requires further investigation.
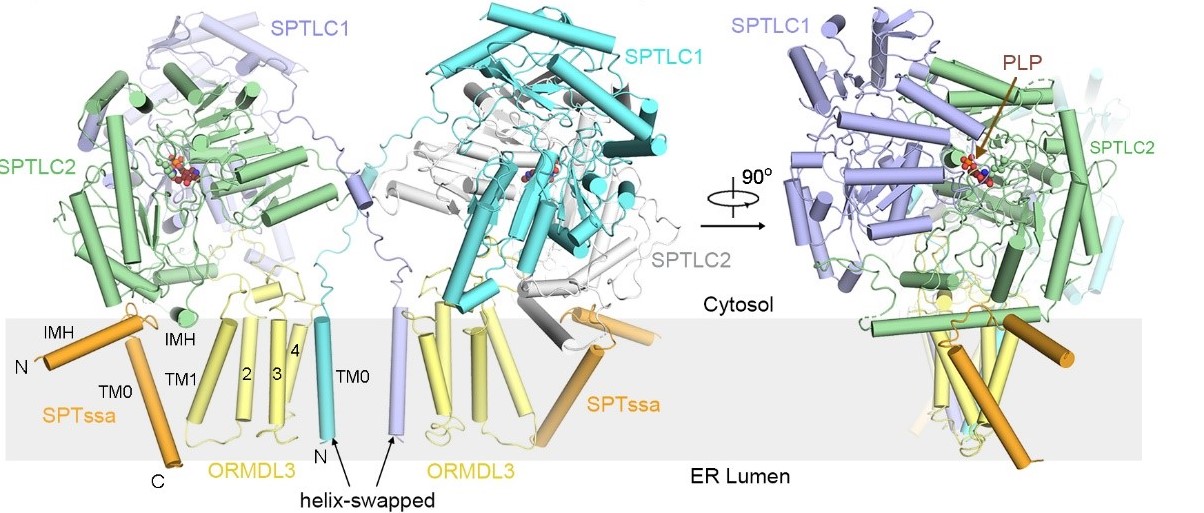
Figure 1. The overall structure of the dimerized SPT-ORMDL3 complex
The study further determined the cryo-EM structure of the SPT-ORMDL3 complex simultaneously bound to serine and a palmitoyl-CoA analog, and verified the substrate-binding site by biochemical methods (Figure 2). The substrate-bound structure reveals a unique structural localization of the residue Met28 of SPTssa, which protrudes into the acyl tail-binding pocket of SPTLC2 (Figure 3a). The Met28 in SPTssa acts as a hydrophobic plug near the distal end of the acyl chain of S-CoA and defines the length of the acyl chain-binding pocket. Consistent with this notion, the substitution of Met28 with valine or glycine, generating SPTssaM28V and SPTssaM28G, respectively, significantly increased the enzymatic activity upon the challenge of the purified SPT-ORMDL3 complex with L-serine and stearoyl-CoA (possessing longer acyl chain than palmitoyl-CoA) substrates (Figure 3b). Despite many remaining questions, the atomic-resolution structures reported in this study establish a new starting point to understand the manner in which sphingolipid homeostasis is regulated by the SPT-ORMDL3 complex and serve as a framework for future studies and drug discovery targeting the complex.
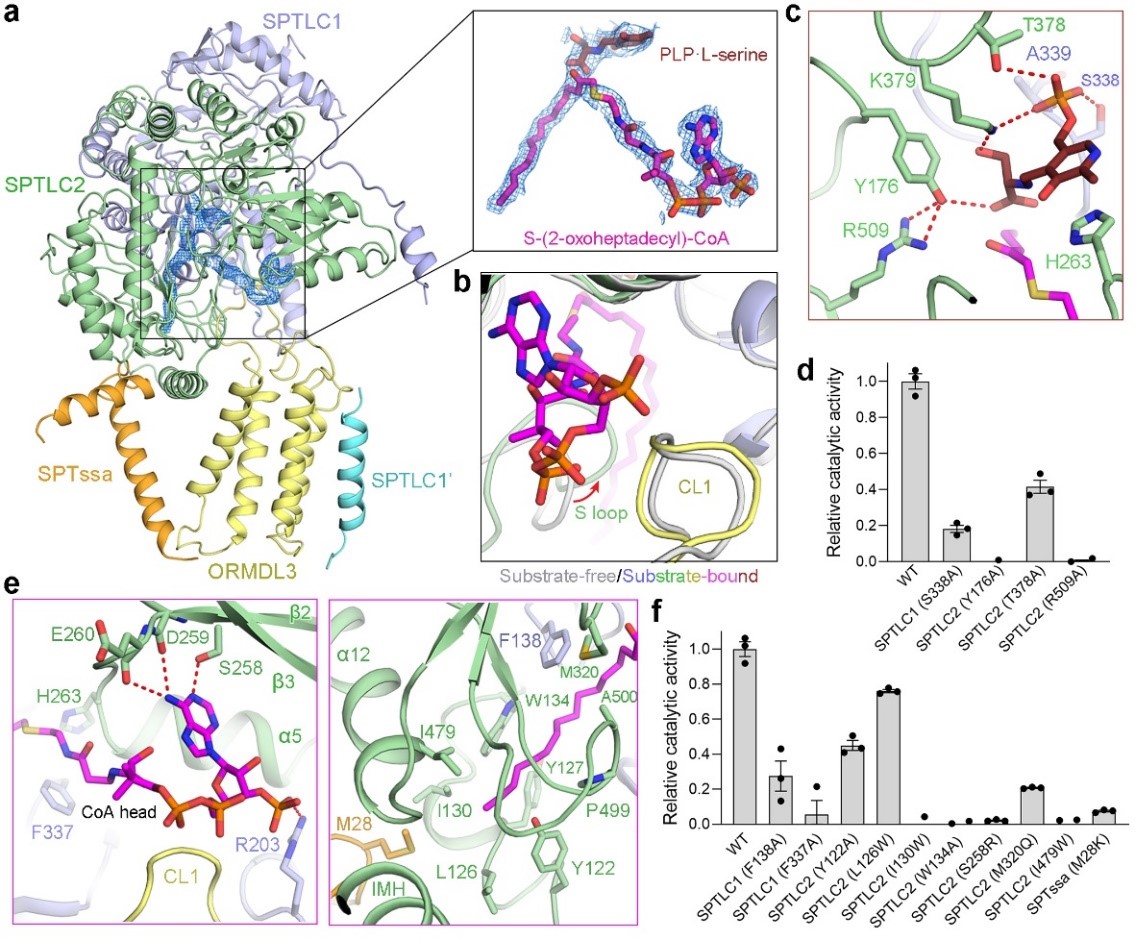
Figure 2. Structure of SPT–ORMDL3 complex bound to two substrates

Figure 3. Acyl-CoA substrate selectivity
This research was accomplished in the Department of Biology of SUSTech. Dr. Xin Gong is the only corresponding author of the paper. Research Associate Professors Sisi Li and Tian Xie, and postgraduate student Peng Liu are the co-first authors of this paper.
This work was supported by the National Natural Science Foundation of China and the Natural Science Foundation of Guangdong Province of China for Distinguished Young Scientists. Cryo-EM data collection and image processing were supported by the cryo-EM center of SUSTech.
Paper link: https://www.nature.com/articles/s41594-020-00553-7
Proofread ByAdrian Cremin, Yingying XIA
Photo By