Sulfilimines, the aza-analogue of sulfoxides, play an important role in the fields of organic synthesis and biomedicine. Several small molecule inhibitors or pesticides of sulfilimines have been developed. However, the synthesis of sulfilimines highly relies on the oxidation conditions, facing shortcomings such as limited functional group compatibility (Figure 1A). Therefore, developing mild and efficient synthetic methods to provide access to sulfilimines with broad functional group tolerance remains a challenge.
Associate Professor Tiezheng Jia’s research team from the Department of Chemistry at the Southern University of Science and Technology (SUSTech) has developed novel and efficient organosulfur chemical synthesis methods using the Chan-Lam coupling reaction as the main tool. Currently, two important research progresses have been published in the Journal of the American Chemical Society (JACS) and Nature Communications.
The team used chemically stable and easily synthesized sulfenamides (R1-S-NH-R2) as the raw material. By introducing carbonyl directing groups into nitrogen atoms, a lower energy and stronger five-membered ring transition state formed with the Cu(II) catalyst. This transition state promoted the S-arylation of sulfenamides and ultimately obtained sulfilimines. This approach overcomes the traditional C-N bond formation of Chan-Lam coupling. This strategy offers several advantages, such as a simple catalytic system, mild reaction conditions, and wide functional group compatibility.
Through theoretical calculations in collaboration with Marisa Kozlowski’s team from the University of Pennsylvania (UPenn), it was found that the high chemical selectivity of the strategy may come from the transmetallation process. The chelation coordination between the S and O atoms of the sulfenamide and the copper catalyst is the key to the lower energy barrier of the transmetallation transition state in the S-arylation pathway.
The research work, entitled “Synthesis of Sulfilimines Enabled by Copper-Catalyzed S‑Arylation of Sulfenamides”, has been published in JACS and received widespread attention from domestic and foreign peers. It is also a highly cited paper.
Dr. Qingjin Liang from the joint doctoral program between SUSTech and the Harbin Institute of Technology (experimental part), and Dr. Lucille Wells from UPenn (theoretical calculation part), are the co-first authors of this paper. Associate Professor Tiezheng Jia and Professor Marisa Kozlowski are the co-corresponding authors. Visiting master’s student Kaiming Han and Professor Shufeng Chen from Inner Mongolia University also made important contributions to this work.
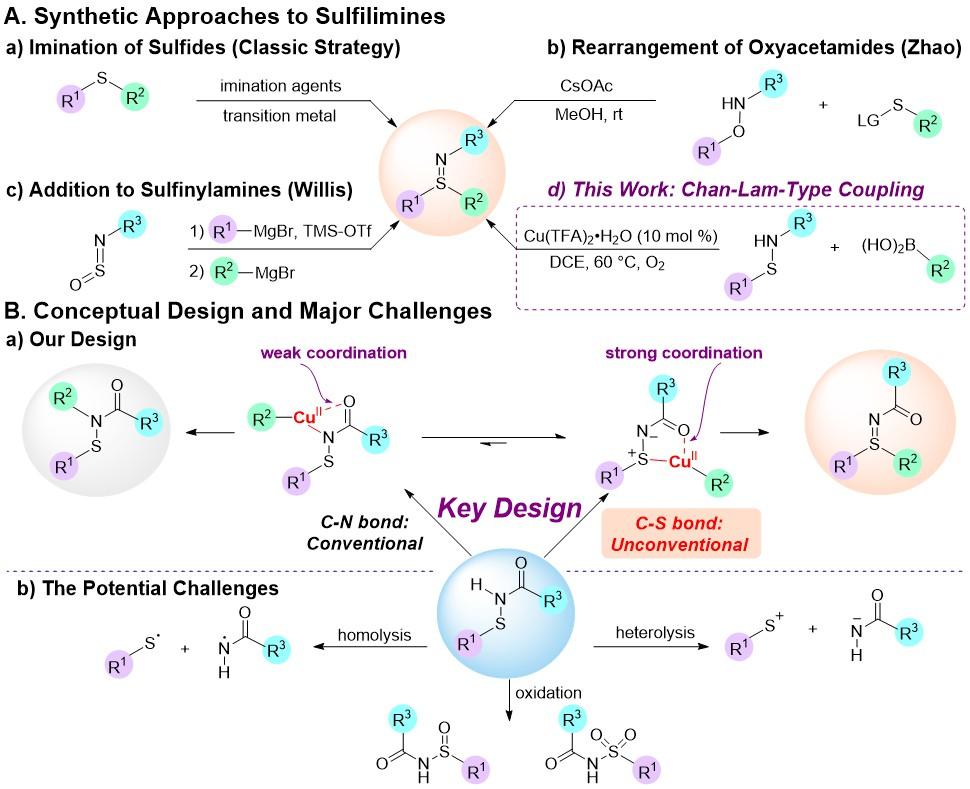
Figure 1. Synthetic approaches to sulfilimines: conceptual design and major challenges
Based on the above work, Tiezheng Jia’s team further expanded the Chan-Lam coupling reaction system of sulfenamide, overcoming the traditional limitation of the Chan-Lam coupling reaction without the need for external ligands. They proposed a new strategy of using ligands to control chemical selectivity in Chan-Lam coupling, and successfully realized the direct N-arylation of NH-sulfenamide to synthesize N-aryl sulfenamide (Figure 2B, a).
Using this new strategy, they prepared N,N-disubstituted sulfenamide, a chemical raw material with important applications in the rubber industry and pharmaceutical chemistry. The strategy has the advantages of simple conditions and economical reaction steps, and can also be applied to direct modification of sulfenamide prodrugs, demonstrating its potential in pharmaceutical chemistry.
Through collaboration with Associate Professor Lizhi Tao from SUSTech, the research team used electron paramagnetic resonance (EPR) technology to study the dynamic behavior of catalysts in the reaction, revealing the single coordination mode between sulfenamide and Cu through N atoms under the action of pybox ligand. Theoretical calculations in collaboration with Professor Marisa Kozlowski indicate that N-arylation under the Pybox/Cu catalytic system is advantageous in both kinetics and thermodynamics, further indicating that the Chan-Lam coupling reaction can become a precise synthesis strategy under ligand control.
The research work, entitled “A combined experimental and computational study of ligand-controlled Chan-Lam coupling of sulfenamides”, has been published in Nature Communications.
Visiting master’s student Kaiming Han, master’s student Hong Liu from SUSTech (experimental part), and Postdoctoral Researcher Madeline Rotella from UPenn (theoretical calculation part) are the co-first authors of this paper. Associate Professor Tiezheng Jia, Professor Marisa Kozlowski, and Professor Shufeng Chen are the co-corresponding authors. Associate Professor Lizhi Tao and master’s student Zeyu Xu from SUSTech also made important contributions to this work.
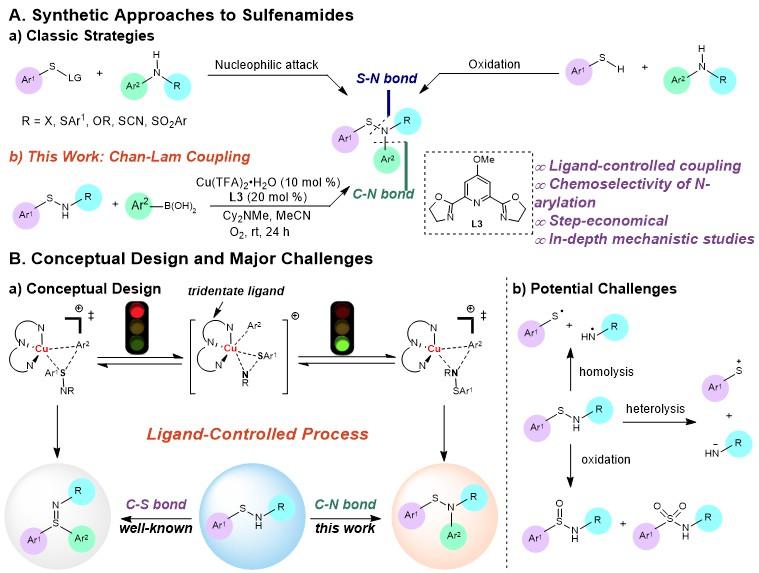
Figure 2. Strategies and challenges in synthesis of sulfenamide
The above research was supported by the National Natural Science Foundation of China (NSFC), Guangdong Basic and Applied Basic Research Foundation, Science and Technology Innovation Commission of Shenzhen Municipality, and the National Institutes of Health (NIH) in the United States.
Paper links (In order of appearance above):
JACS: https://doi.org/10.1021/jacs.2c12947
Nature Communications: https://www.nature.com/articles/s41467-024-49089-0
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo ByDepartment of Chemistry