The nuclear matrix, as an important three-dimensional network structure within the cell nucleus, is composed of proteins, RNA, and other molecules. It plays crucial roles in supporting the shape of the nucleus, regulating the spatial organization of chromatin, promoting gene expression, and determining cell fate.
During early embryonic development, cells gradually transition from a totipotent state to a pluripotent state and finally differentiate into specific lineages. This process is accompanied by the remodeling of the chromatin structure and dynamic changes in epigenetic regulation. However, how the nuclear matrix regulates cell fate during this process—particularly its specific mechanisms in the early stages of development—remains an unsolved mystery.
A research team led by Associate Professor Andrew P. Hutchins from the Department of Systems Biology, School of Life Sciences at the Southern University of Science and Technology (SUSTech), in collaboration with Associate Professor Yiming Li from the Department of Biomedical Engineering at SUSTech and Professor Dongwei Li from Guangzhou Medical University, has revealed a mechanism by which the nuclear matrix maintains the state of pluripotent stem cells. Their study elucidates how the nuclear matrix network, regulated by heterogeneous nuclear ribonucleoprotein U (HNRNPU), stabilizes genes associated with the primed state in pluripotent stem cells.
Their paper, titled “The nuclear matrix stabilizes primed-specific genes in human pluripotent stem cells”, has been published in the journal Nature Cell Biology.
The research team found that by reducing the expression levels of HNRNPU or the nuclear matrix protein Matrin-3 (MATR3) causes primed human pluripotent stem cells (hPSCs) to spontaneously revert to an earlier pluripotent state. This reversion is characterized by changes in cell morphology akin to the naïve state, as well as the upregulation of naïve pluripotency marker genes. Moreover, reducing HNRNPU expression can promote somatic cell reprogramming and facilitate the generation of totipotent stem cells.
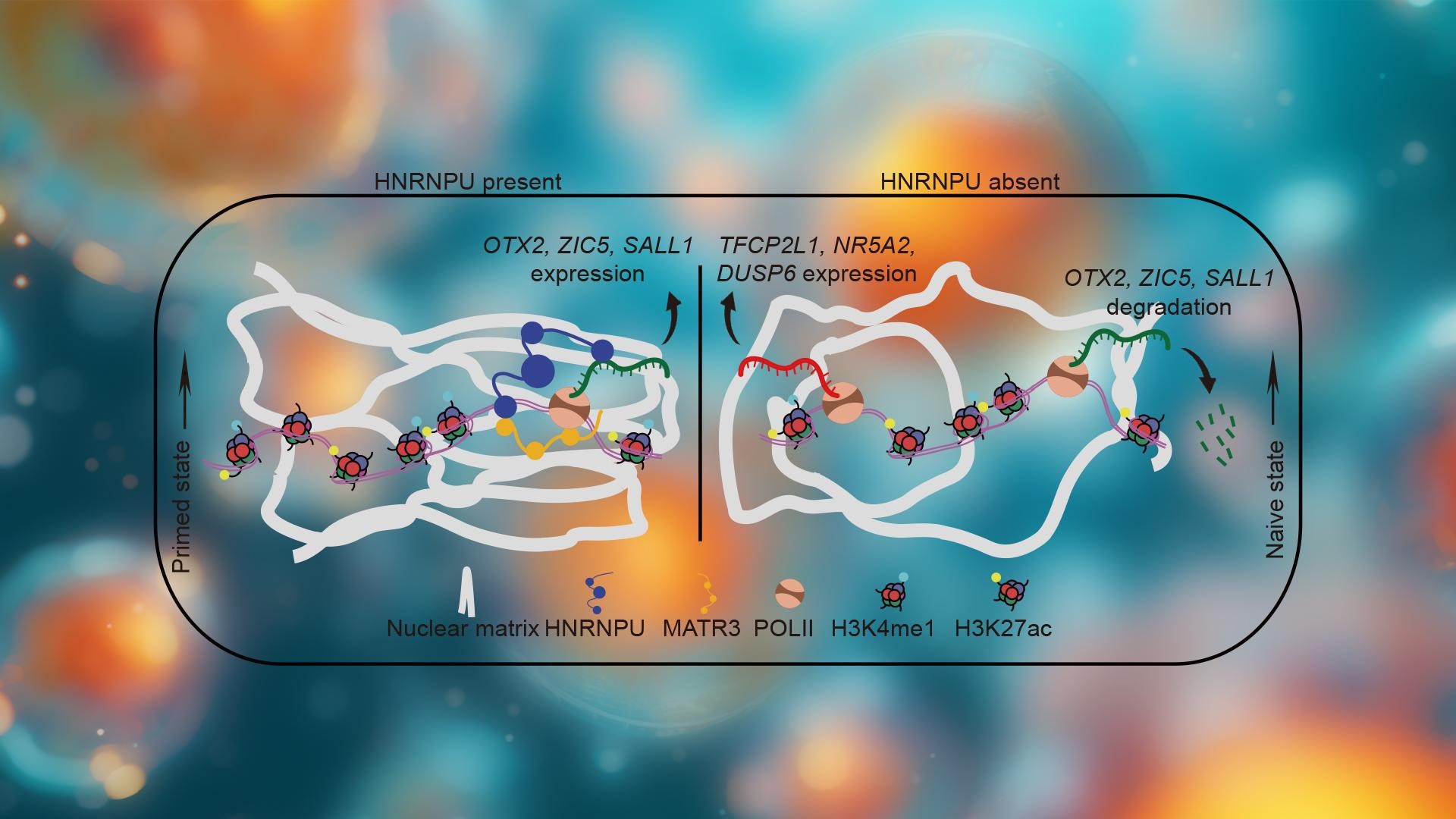
To further investigate how the nuclear matrix regulates the three-dimensional genome structure to control cell identity and modulate pluripotency states, the researchers combined genomic techniques with super-resolution imaging. They found that HNRNPU forms a dense network within the cell nucleus, which colocalizes with chromatin, RNA polymerase II (POL II), and the nuclear matrix through MATR3. Furthermore, reducing HNRNPU levels affects the physical properties of chromatin, including chromatin relaxation, decreased three-dimensional chromatin interactions, and an increase in nuclear volume. These observations suggest that the nuclear matrix plays a crucial role in maintaining nuclear morphology and genome integrity. At the same time, such changes may create favorable conditions for the reactivation of naïve pluripotency genes, thereby promoting a shift of cells to an earlier developmental state.
Further research results show that HNRNPU, acting as a key nuclear matrix anchoring protein, links the promoter regions of primed-specific genes to scaffold/matrix attachment regions (SARs). In doing so, it regulates RNA polymerase II (POL II) transcription elongation and maintains RNA stability. Through its synergistic interaction with POL II, HNRNPU anchors these genes to the nuclear matrix, promoting their transcription elongation and stabilizing their RNA. HNRNPU can also directly bind to nascent RNA molecules, modulating the transcription elongation process and half-life of primed-specific gene transcripts. The study found that when HNRNPU levels decrease, transcription elongation of these genes is significantly reduced, and their RNA stability markedly diminishes. This finding further underscores the critical role of HNRNPU in maintaining RNA stability.
Overall, HNRNPU plays a crucial role in maintaining cell type stability. When its expression level is reduced, cells tend to transition to an earlier pluripotent state. This finding offers a new perspective on the state transitions of pluripotent stem cells and the role of the nuclear matrix in regulating cell fate, which holds significant implications for regenerative medicine and developmental biology research. Moreover, the study’s results provide new clues for understanding nuclear matrix-related diseases. Further exploration of the functions of the nuclear matrix in different cell types and disease contexts may offer new directions for future diagnostic and therapeutic strategies.
Ph.D. students Gang Ma and Xiuling Fu (currently a postdoctoral researcher at Columbia University), as well as postdoctoral researcher Lulu Zhou, all from SUSTech, are the co-first authors of the paper. Associate Professor Andrew P. Hutchins, Associate Professor Yiming Li, and Professor Dongwei Li are the corresponding authors.
Paper link: https://www.nature.com/articles/s41556-024-01595-5
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo ByYan QIU