Archaea, one of Earth’s most ancient microbial groups, thrive in extreme environments such as high temperatures, acidity, and salinity. Their unique cell membranes, composed primarily of ether-linked lipids like glycerol dialkyl glycerol tetraether (GDGT), enable survival under harsh conditions. While GDGT cyclization, involving the insertion of cyclopentane or cyclohexane rings on the biphytanyl chains, is known to regulate membrane rigidity and fluidity, its physiological functions and regulatory mechanisms remain unclear.

A collaborative research team led by Associate Professor Zhirui Zeng from the Department of Ocean Science and Engineering at the Southern University of Science and Technology (SUSTech) has provided the first systematic elucidation of how GDGT cyclization in the membrane lipids of thermophilic archaea modulates membrane protein function and cell motility. Their study reveals a molecular strategy by which archaea adapt to extreme environments through dynamic membrane lipid remodeling.
Their findings, titled “Cyclization of Archaeal Membrane Lipids Impacts Membrane Protein Activity and Archaellum Formation”, have been published in the Proceedings of the National Academy of Sciences (PNAS).
Using Sulfolobus acidocaldarius, a model thermophilic archaeon, the research team combined genetic manipulation, proteomics, and metabolic pathway analysis to demonstrate that disrupting GDGT cyclization inhibits archaellum formation and significantly reduces cell motility (Figure 1). Further investigation revealed that GDGT cyclization regulates the stability of the transmembrane protein ArnR/R1, which indirectly modulates the activity of transcription factors critical for archaellum gene expression. Additionally, reduced GDGT cyclization decreased the expression of the respiratory complex SoxABCD but promoted the expression of the SoxEFGHIM supercomplex, suggesting that membrane lipid modifications remodulate energy metabolism to cope with environmental stress. The widespread co-occurrence of GDGT ring synthases (GrsA/B) and archaellum-related proteins across thermophilic archaea hints at a universal mechanism linking membrane structure to cellular function.
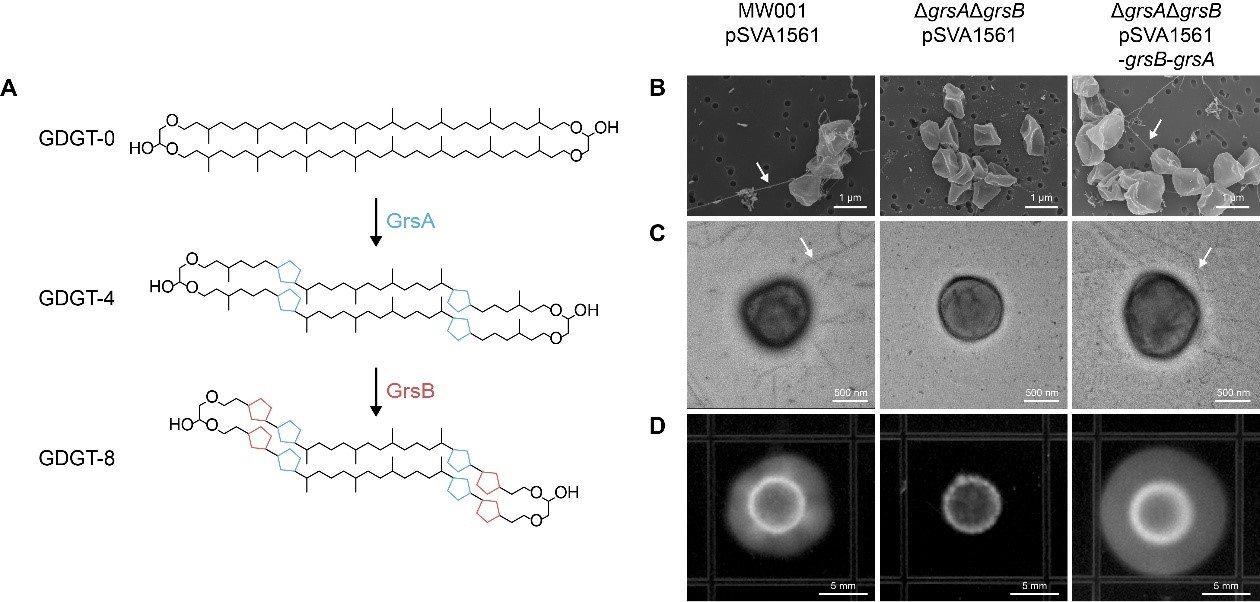
Figure 1. Loss of GDGT cyclization in archaeal cell membrane inhibits archaellum formation and impairs cell motility.
This study established a direct link between archaeal membrane lipid modifications and cell motility for the first time, revealing the core role of dynamic membrane lipid remodeling in adaptation to extreme environments. It points out that GDGT cyclization is not only a physical barrier for archaea to cope with temperature changes, but also a “molecular switch” that finely regulates cell functions through a signal transduction network.
The discovery offers a new perspective for understanding the functional diversity of archaeal membrane lipids. This research provides a theoretical basis for the development of microbial resources in extreme environments and the design of artificial membranes in synthetic biology, and has certain significance for the study of the early evolution of life on Earth.
Senior Research Scholar Wei Yang from Zhirui Zeng’s Lab at SUSTech is the first author of the paper. Associate Professor Zhirui Zeng and Dr. Changyi Zhang from the Carl R. Woese Institute for Genomic Biology at the University of Illinois Urbana-Champaign (UIUC) are the corresponding authors. Other contributors to this study include Ph.D. students Xi Feng and Huahui Chen from SUSTech, and Professor Thomas J. Santangelo and Dr. Geraldy Liman from Colorado State University.
Paper link: http://doi.org/10.1073/pnas.2423648122
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yifei REN
Photo ByYan QIU