Fungal infections impact over a billion individuals worldwide and contribute to more than 1.6 million fatalities annually, posing a substantial global health concern. Given the widespread prevalence of fungal infections, the limited options for antifungal drugs, and the rapid evolution of drug-resistant fungi, there is an urgent need to identify novel therapeutic targets and develop new antifungal medications.
One promising target is inositol phosphorylceramide (IPC) synthase, an enzyme complex essential for fungal survival and virulence. Consisting of catalytic subunit Aur1 and regulatory subunit Kei1, IPC synthase does not exist in mammals, making it a highly attractive target for therapeutic intervention against fungal infections. A natural cyclic depsipeptide antifungal antibiotic named aureobasidin A (AbA) has been reported to inhibit the fungal IPC synthase at sub-nanomolar concentrations. However, the lack of a resolved structure for the IPC synthase complex has limited our understanding of the precise functional roles of the Aur1 and Kei1 subunits within the complex and constrained our ability to rationalize drug-resistance mechanisms of the mutations.
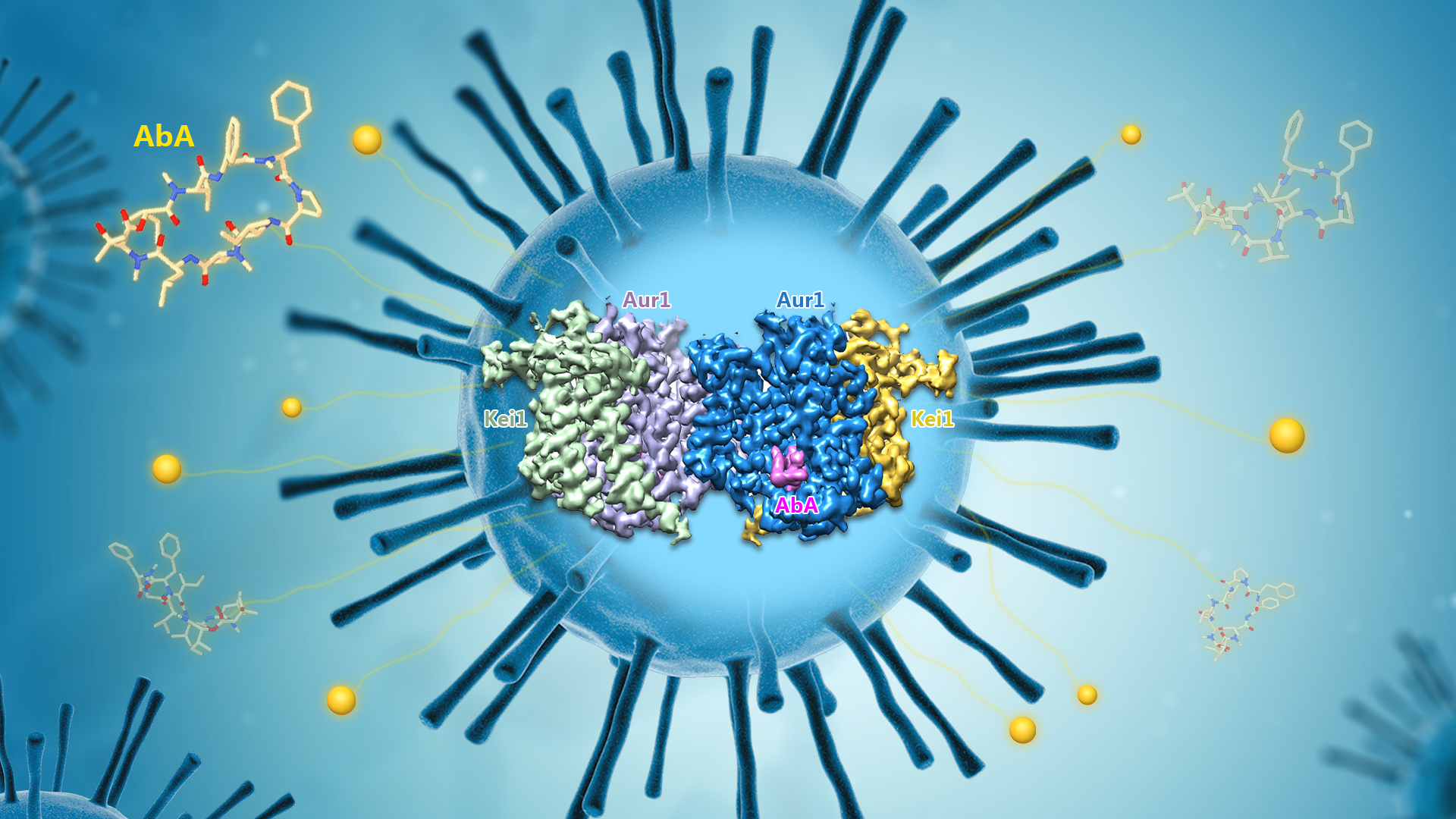
Associate Professor Xin Gong’s research team from the School of Life Sciences at the Southern University of Science and Technology (SUSTech) has, for the first time, resolved the high-resolution cryo-electron microscopy structure of the IPC synthase complex bound to the specific inhibitor AbA. Their work elucidated the molecular mechanisms underlying AbA’s inhibitory effects and drug resistance, providing a foundation for the development of therapeutic drugs targeting fungal IPC synthase.
Their paper, titled “Mechanisms of aureobasidin A inhibition and drug resistance in a fungal IPC synthase complex”, has been published in the international journal Nature Communications.
In this study, the researchers obtained the S.cerevisiae Aur1-Kei1 complex through in vitro recombinant overexpression and purification, and successfully resolved a 3.17 Å cryo-EM structure of the Aur1-Kei1 complex bound to AbA. Subsequent in vitro biochemical activity assays validated the specific mechanism of inhibitor binding. The three-dimensional structure revealed that yeast IPC synthase forms into a tetrameric complex, consisting of two Aur1-Kei1 heterodimers. The two Aur1 catalytic subunits are centrally located and form a homodimer, while each Kei1 regulatory subunit is positioned at each end (Figure 1). Although Kei1 does not directly participate in catalysis, it is essential for the proper assembly and enzymatic activity of the complex.
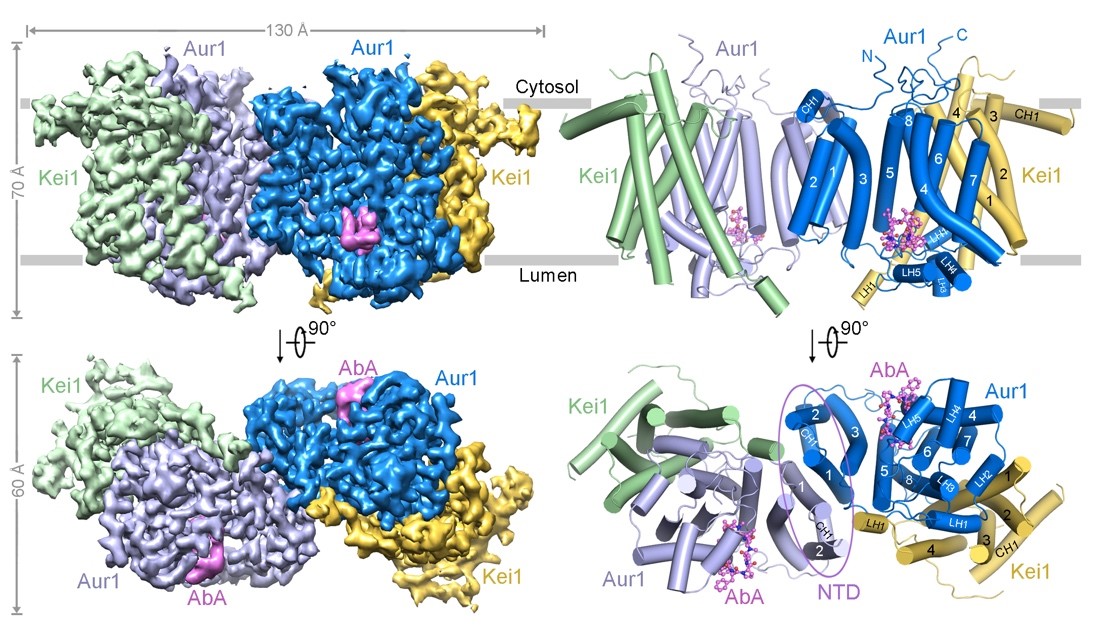
Figure 1. Overall structure of the AbA-bound IPC synthase complex
Structural and biochemical analyses revealed that AbA inhibits enzyme activity by binding near the substrate-binding pockets, thereby blocking the substrate PI and ceramide entry (Figure 2, left). Conservation analysis reveals that the residues within the AbA binding pocket are highly conserved across fungi, which aligns well with the broad antifungal spectrum of AbA. Mutations conferring resistance to AbA are clustered around the AbA-binding pocket, indicating that direct disruption of AbA binding underlies the mechanism of resistance (Figure 2, right).
This work not only reports the first three-dimensional structure and functional mechanism of the fungal IPC synthase, but also reveals the inhibitory mechanism of the natural antibiotic AbA and the molecular basis of drug resistance. The AbA-binding pocket resolved in this study provides a foundation for optimizing AbA derivatives to enhance antifungal efficacy or avoid drug resistance. It could greatly accelerate the development and optimization of antifungal therapeutics targeting fungal IPC synthase.
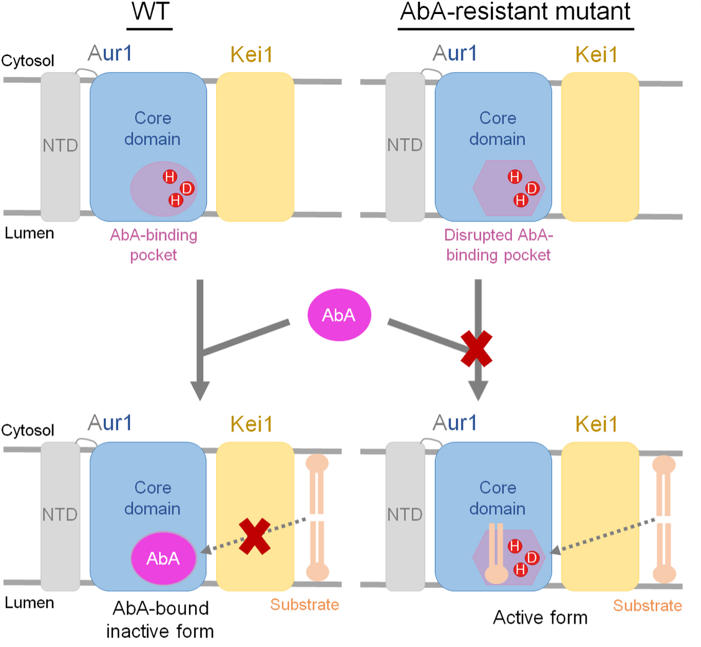
Figure 2. A working model of inhibition and drug-resistance mechanism of AbA towards IPC synthase
Ph.D. candidate Xinyue Wu from the School of Life Sciences at SUSTech is the first author of the paper. Associate Professor Xin Gong and Research Associate Professor Tian Xie are the co-corresponding authors, with SUSTech serving as the primary affiliated institution.
Paper link: https://www.nature.com/articles/s41467-025-60423-y
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yifei REN
Photo BySchool of Life Sciences