The research group led by Associate Professor Liu Leo LIU from the Department of Chemistry, College of Science, Southern University of Science and Technology (SUSTech), has achieved a breakthrough in the field of main-group aluminum chemistry. The research findings, entitled “Aluminum redox catalysis enables cyclotrimerization of alkynes,” were published in the international top-tier journal Nature. This work represents the first realization of redox catalysis mediated by aluminum, laying an important foundation for the systematic development of aluminum chemistry and significantly expanding the conceptual understanding of catalysis by main-group elements, establishing an entirely new research paradigm.
Transition-metal catalysis has made the efficient construction of complex molecules possible and has had a profound impact on modern chemistry and societal development. This capability stems from the unique electronic structures of transition metals, which typically possess energetically similar, partially occupied valence d orbitals. These orbitals can be finely tuned through changes in the coordination environment during a reaction, allowing transition metals to both donate and accept electrons. As a result, they can smoothly interconvert between different oxidation states and coordination numbers, enabling key elementary steps such as oxidative addition, ligand exchange, migratory insertion, and reductive elimination to selectively form and break chemical bonds. In contrast, main-group compounds generally feature valence s and p orbitals that are either fully occupied or empty, with larger energy separations and stronger directionality. Their interactions with small molecules are therefore often weaker, and their limited availability of low-energy vacant orbitals and coordination flexibility makes it difficult to stabilize and recycle key intermediates. Consequently, reversible electron transfer between low and high valence states, and thus redox catalysis, has historically been rare for main-group elements. Notably, recent advances in ligand design and electronic-structure adjusting are rapidly expanding the boundaries of main-group redox catalysis.
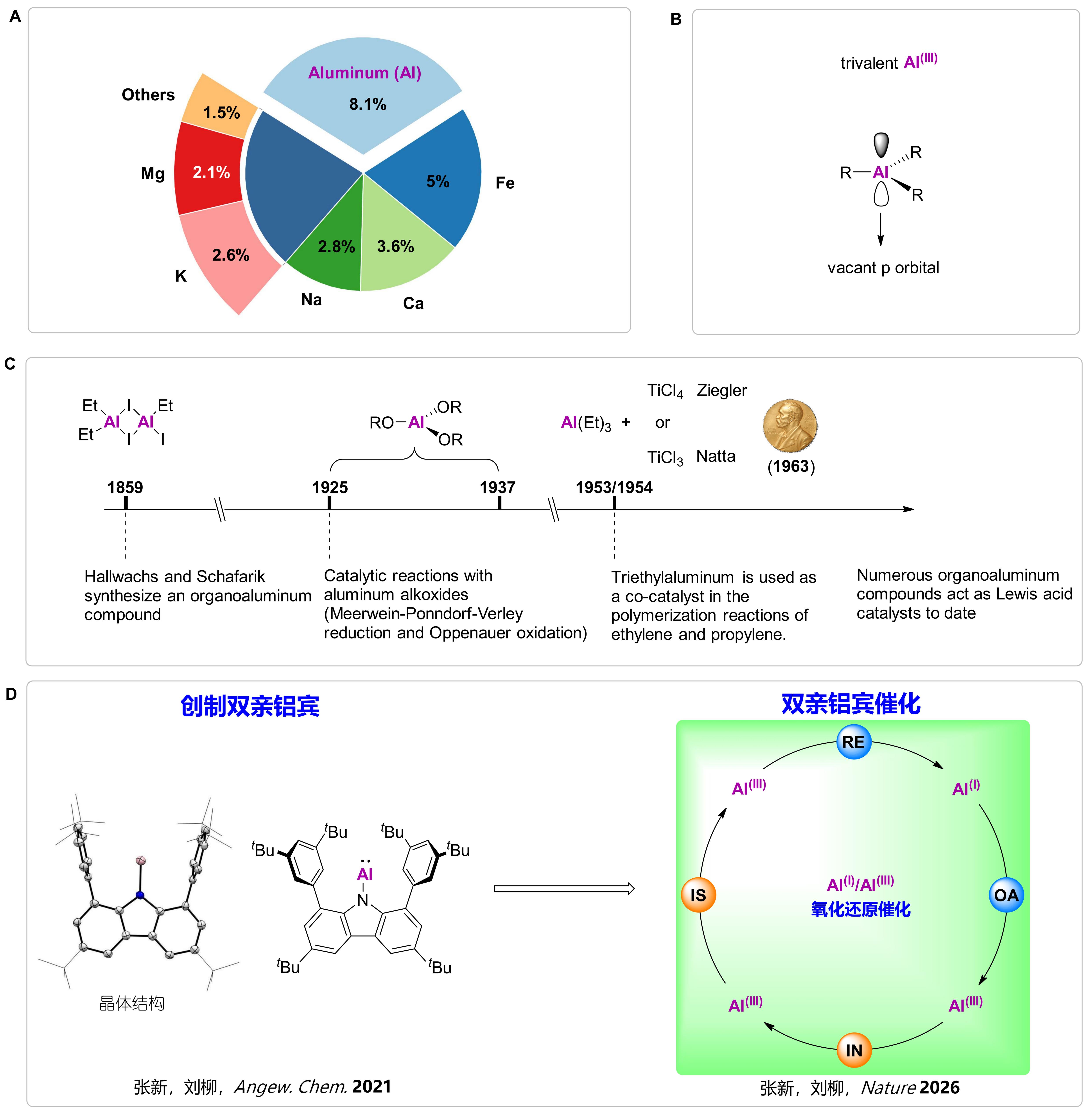
Figure 1. A. Elemental abundance in the Earth’s crust; B. Electronic structure of Al(III) compounds; C. Key milestones in Al(III) catalysis; D. Overview of the present work.
Aluminum is the most abundant metallic element in the Earth’s crust (8.1%; Figure 1A). In the periodic table, aluminum is not only the first metallic element in Group 13 but also the first metal in the p-block, with a valence electron configuration of 3s²3p¹. As a third-period element, aluminum is essentially unaffected by relativistic effects and possesses the lowest electronegativity among p-block elements (1.61). Governed by these intrinsic electronic features, aluminum strongly favors the loss of three valence electrons to form thermodynamically highly stable trivalent aluminum compounds (Figure 1B). In such Al(III) species, the aluminum center retains an empty 3p orbital, endowing them with pronounced Lewis acidity. Since the synthesis of the first organoaluminum compound in 1859, applications of aluminum compounds in catalysis over nearly 170 years have been largely confined to Al(III)-based Lewis acid catalysis (Figure 1C).
Over the past three decades, isolable low-valent aluminum(I) compounds have remained exceedingly rare. Al(I) species possess frontier orbitals that simultaneously contain a lone pair and empty orbitals, exhibiting typical Lewis ambiphilicity (both nucleophilic and electrophilic). As a result, they readily undergo irreversible oxidative reactions with small molecules, rapidly converting into thermodynamically stable Al(III) compounds. This inherent reactivity has long posed a formidable challenge to establishing a viable redox cycle for aluminum.
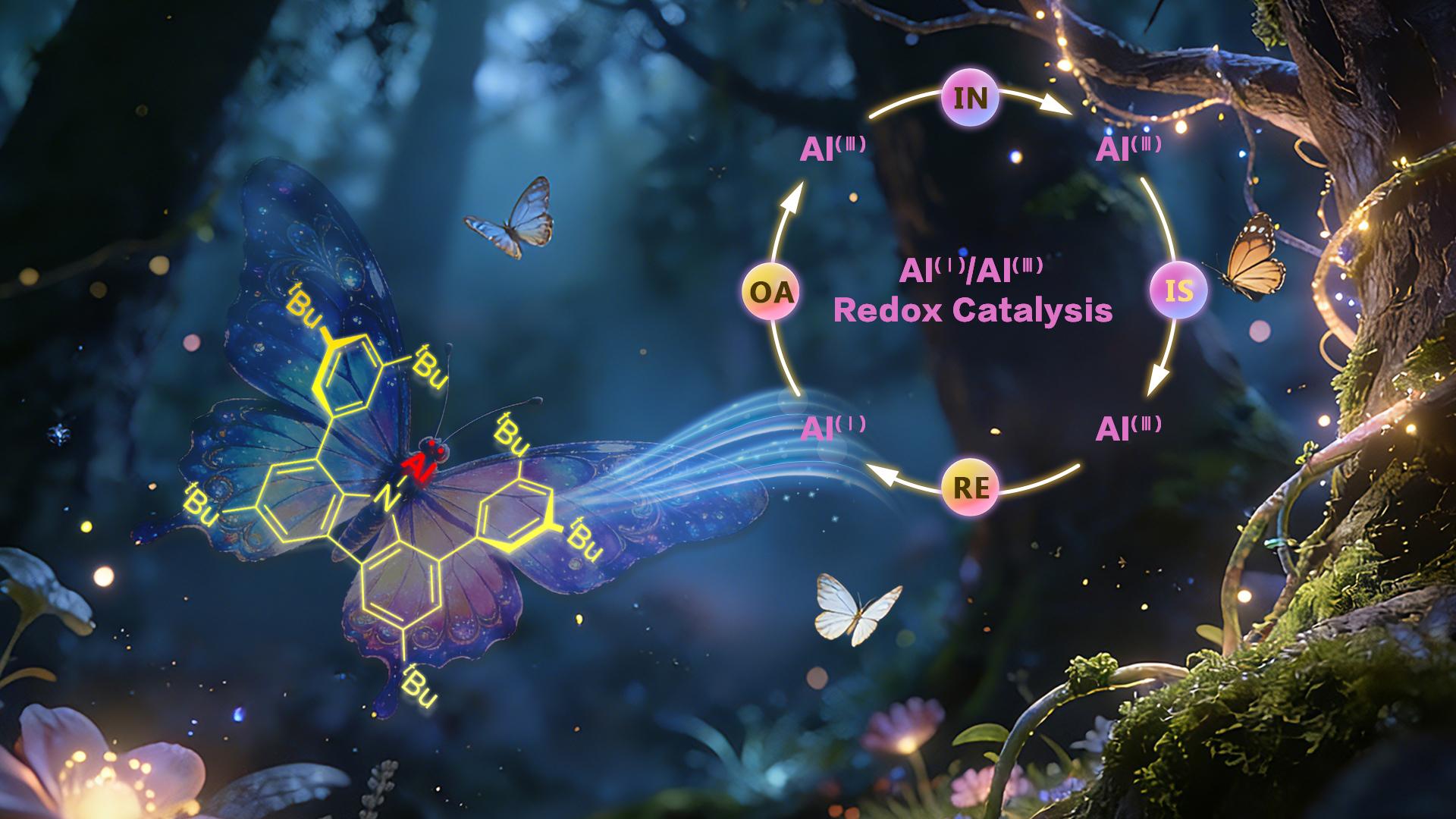
In 2021, the Liu Leo LIU group designed and isolated an aluminylene, an aluminum congener of carbenes (Figure 1D; the group’s first independent work at SUSTech, Angew. Chem. Int. Ed. 2021, 60, 27062). The team ingeniously exploited the unique “tri-active ambiphilicity” of this aluminyl species to couple oxidative addition and insertion reactions of alkynes. Simultaneously, the “conformational adaptability” of the nitrogen atom within the carbazolyl substituent was harnessed to facilitate key isomerization and reductive elimination steps. Together, these features enabled the construction of an Al(I)/Al(III)-based redox catalytic cycle, culminating in the successful realization of a Reppe-type alkyne cyclotrimerization reaction (Figures 1D and 2A).
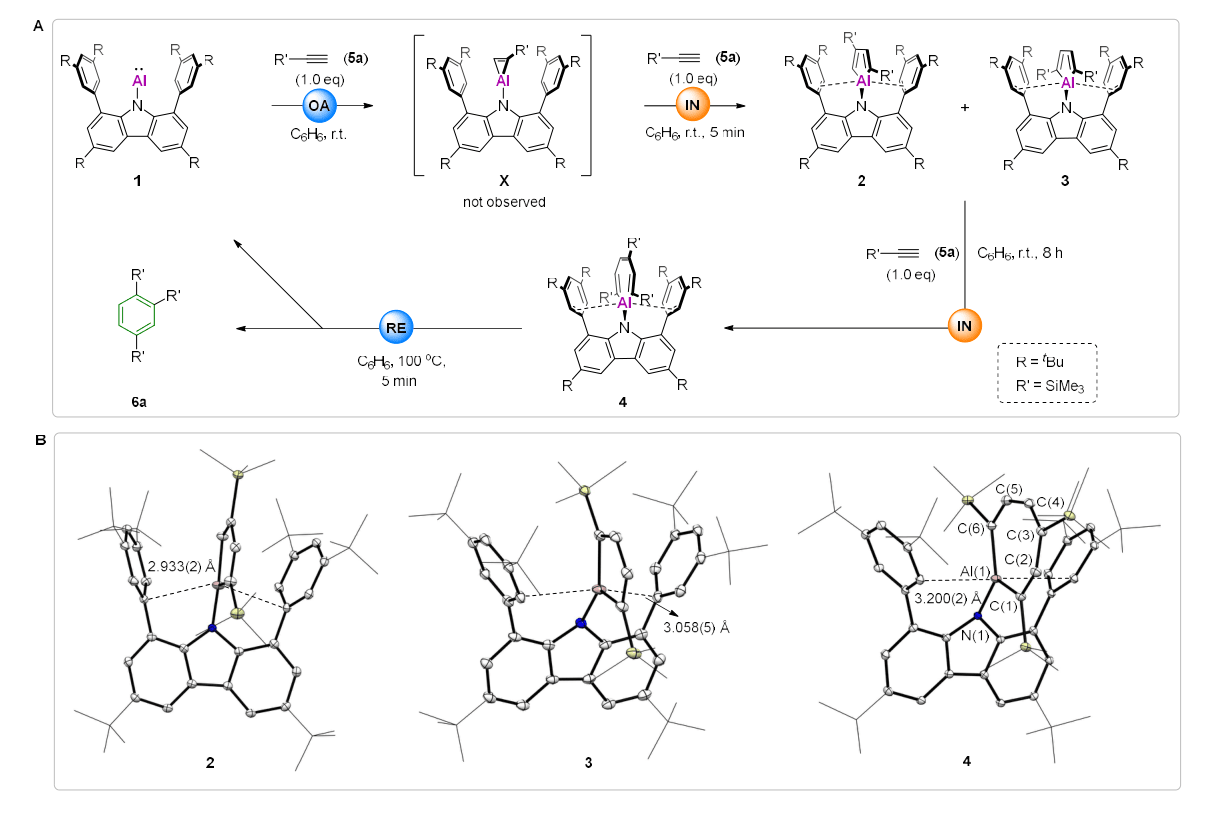
Figure 2. Stoichiometric reactions of the aluminyl species with alkynes and crystal structures of key intermediates.
All three key intermediates in the catalytic cycle were unambiguously characterized by single-crystal X-ray diffraction (Figure 2B). Kinetic studies and theoretical calculations further support a complete Al(I) → Al(III) → Al(I) redox cycle. Notably, the catalytic system exhibits excellent functional group tolerance and outstanding chemoselectivity (Figure 3A), as well as remarkably high catalytic activity, with turnover numbers reaching up to 2290 (Figure 3B). Compared with previously reported non-precious-metal systems for alkyne cyclotrimerization, aluminum catalysis demonstrates significantly superior chemoselectivity relative to molybdenum-, iron-, and cobalt-based catalysts.
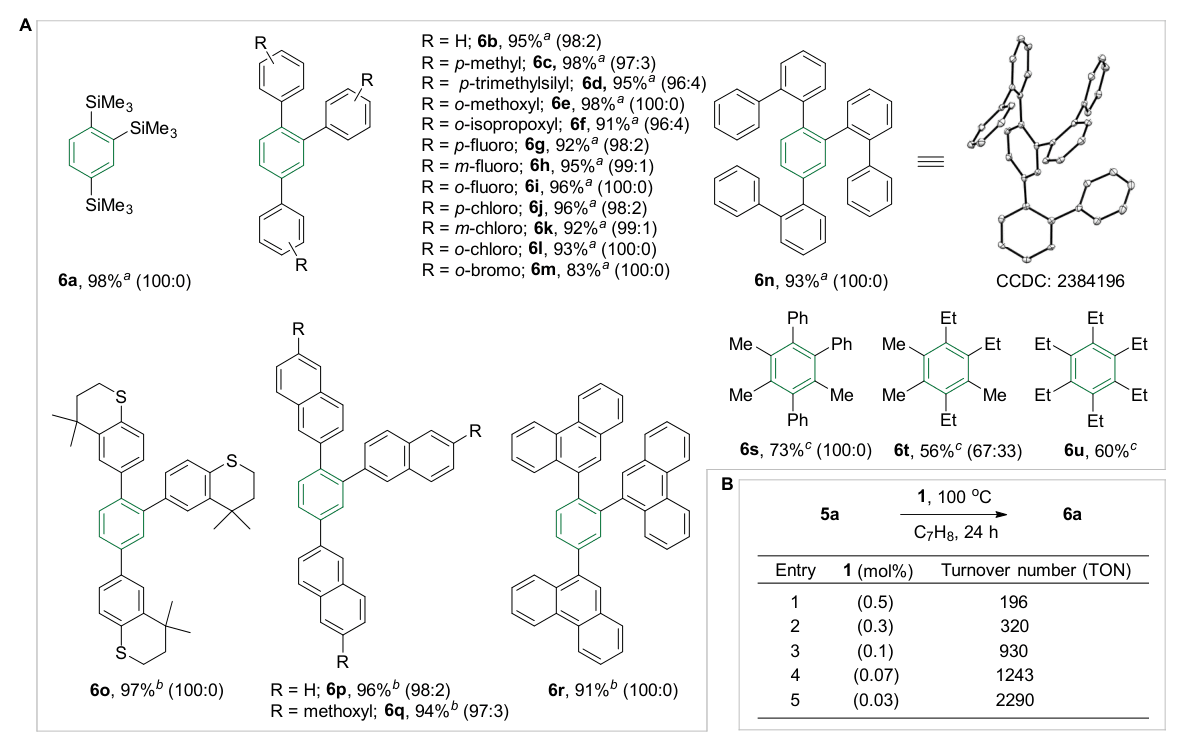
Figure 3. A. Substrate scope; B. Determination of turnover numbers.
This work not only fills a longstanding gap in the field of aluminum redox catalysis but also deepens the fundamental understanding of aluminum bonding characteristics and reaction mechanisms. It provides an important foundation and new direction for the future development of aluminum chemistry and related main-group element chemistry.
The Liu Leo LIU group has been devoted to research in elemental and organometallic chemistry, with a focus on the design and transformation of ambiphilic main-group molecules. With SUSTech as the corresponding institution, the group has published a paper in Science, CCS Chemistry, Nature, and Nature Chemistry each; two in Nature Synthesis, Chem, and Nature Communications each; nine papers in the Journal of the American Chemical Society, and 12 in Angewandte Chemie International Edition. Several of these works have been highlighted by Science, Nature Synthesis, Chem, ChemistryViews, and ChemistryWorld as Editors’ Choice, Highlights, or Previews.
This study was conducted solely at the Department of Chemistry, SUSTech. Associate Professor Liu Leo LIU is the corresponding author, and Dr. Xin ZHANG, senior research scholar in the Department of Chemistry, is the first author.
In the same issue of Nature, the paper was highlighted and featured in the News & Views section under the title “Untapped catalytic ability of aluminium has been unlocked.”
Article link: https://www.nature.com/articles/s41586-025-09941-9
News & Views link: https://www.nature.com/articles/d41586-026-00174-y
Proofread ByNoah Crockett, Junxi KE
Photo ByYan QIU