Professor Xin-Yuan LIU’s research team from the Department of Chemistry at the Southern University of Science and Technology (SUSTech) has achieved progress in the asymmetric construction of chiral all-carbon quaternary stereocenters. The related findings, entitled “Copper-catalysed enantioconvergent radical C(sp3)–C(sp3) cross-coupling of tertiary electrophiles with cyclopropanols for quaternary carbon formation,” have been published in Nature Synthesis, one of the most preeminent academic journals in chemistry.
Chiral all-carbon quaternary stereocenters are widely found in natural products, agrochemicals, and pharmaceutical molecules. They are highly valuable yet challenging targets in modern organic synthesis due to their ability to confer higher metabolic stability, conformational rigidity, and target selectivity to molecules. Transition-metal-catalyzed enantioconvergent radical cross-coupling of racemic tertiary alkyl electrophiles with alkyl nucleophiles represents one of the most attractive strategies for constructing such motifs. However, related reports are extremely scarce. The core challenges lie in: the difficulty in controlling chemoselectivity and enantioselectivity due to the hindered interaction between bulky tertiary alkyl radicals and chiral transition metal-alkyl species; concurrently, the in-situ generated transition metal-alkyl species are prone to side reactions such as β-hydride elimination, dimerization of alkyl nucleophiles, and proto-demetallation, while the highly reactive tertiary alkyl radicals tend to undergo hydrogen atom transfer or disproportionation.
Professor LIU’s group has long been dedicated to research on radical asymmetric catalysis. In recent years, they have developed a series of copper-catalyzed enantioconvergent radical cross-coupling reactions. To address the aforementioned challenges, the team conceived employing stable and readily accessible cyclopropanols as C(sp3) nucleophiles, whose strain-induced ring-opening would generate Cu-homoenolate intermediates. Meanwhile, leveraging the inherent low propensity for β-hydride elimination of copper catalysts, combined with the team’s recently developed polydentate anionic chiral ligand/copper catalytic system, offered promise for stabilizing the in-situ formed coordination-saturated copper-homoenolate intermediate and suppressing its decomposition. Based on this rationale, the team successfully developed a practical copper-catalyzed enantioconvergent C(sp3)–C(sp3) cross-coupling reaction.
The key to this reaction was the rational design of a class of cinchona alkaloid skeleton-derived anionic amide N,N,N-tridentate chiral ligands. The intermediate formed by these ligands with copper features reduced steric congestion around the metal center, enabling it to accommodate bulky tertiary alkyl radicals. This design successfully achieved excellent chemoselectivity and enantioselectivity control under mild conditions. This strategy provides an efficient method for the direct synthesis of chiral all-carbon quaternary stereocenters bearing a remote γ-carbonyl functional group.
The reaction demonstrates a broad substrate scope. Various aryl-, heteroaryl-, and alkyl-substituted cyclopropanols proved to be effective alkyl nucleophiles. Furthermore, diverse tertiary electrophiles, such as α-bromo-γ-lactams and α-bromo-β-lactams, underwent smooth coupling to afford the desired products in excellent yields and with high enantioselectivity. Derivatives of bioactive molecules like probucol, indomethacin, naproxen, and lithocholic acid also reacted successfully, highlighting the potential of this method for complex molecule modification. Moreover, the coupling products could be further transformed into various synthetically challenging chiral quaternary carbon building blocks, such as chiral γ-lactams and β- or γ-amino acid derivatives, with the enantiopurity of the stereocenter retained throughout these processes.
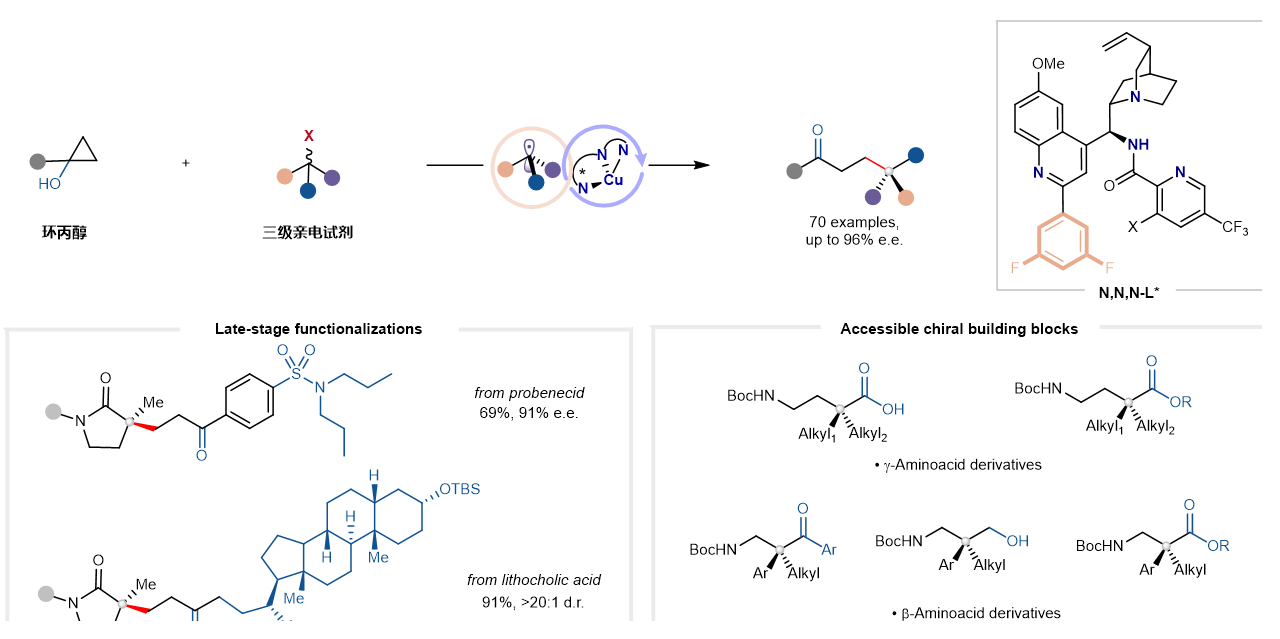
Figure. Copper-catalyzed enantioconvergent quaternization reaction based on cyclopropanol ring-opening.
This study represents the first successful highly enantioselective copper-catalyzed C(sp3)–C(sp3) cross-coupling of racemic tertiary electrophiles with alkyl nucleophiles. It overcomes the long-standing challenges of chemical and stereoselective control with bulky tertiary alkyl substrates in such reactions, offering a highly flexible and practical platform for rapidly constructing structurally diverse, C(sp3)-rich chiral quaternary architectures. This strategy is expected to provide new insights for catalyst design and development in asymmetric cross-coupling reactions involving more hindered electrophiles and nucleophiles.
Research Assistant Professor Dong GAO and Dr. Long-Zhou QIN from the Department of Chemistry at SUSTech are the co-first authors of the paper. Chair Professor Xin-Yuan LIU is the corresponding author. SUSTech is the first affiliation.
Proofread ByNoah Crockett, Junxi KE
Photo ByYan QIU