On Oct 2 2017, the Department of Chemistry’s Associate Professor Bin Tan and his research group have made a significant advance in the field of asymmetric organocatalysis by reporting the discovery of a new function of the azo group, enabling chiral phosphoric acid-catalyzed asymmetric aryl carbon–hydrogen functionalization.
The research, entitled with Organocatalytic asymmetric arylation of indoles enabled by azo groups, was recently published online in Nature Chemistry (Impact Factor = 27.89).Arylation is a fundamental reaction that can be mostly fulfilled by electrophilic aromatic substitution and transition-metal-catalysed aryl functionalization. Although the azo group has been used as a directing group for many transformations via transition-metal-catalysed aryl carbon–hydrogen (C–H) bond activation, there remain significant unmet challenges in organocatalytic arylation.
Here, we show that the azo group can effectively act as both a directing and activating group for organocatalytic asymmetric arylation of indoles via formal nucleophilic aromatic substitution of azobenzene derivatives. Thus, a wide range of axially chiral arylindoles have been achieved in good yields with excellent enantioselectivities by utilizing chiral phosphoric acid as catalyst. Furthermore, highly enantioenriched pyrroloindoles bearing two contiguous quaternary chiral centres have also been obtained via a cascade enantioselective formal nucleophilic aromatic substitution–cyclization process. This strategy should be useful in other related research fields and will open new avenues for organocatalytic asymmetric aryl functionalization.
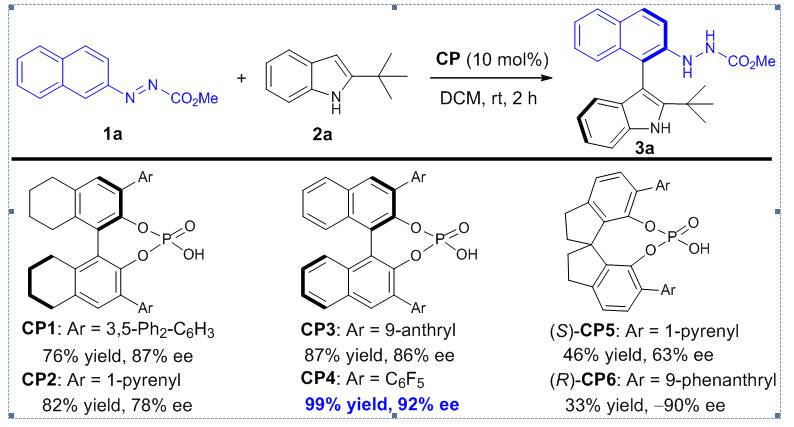
Figure 1 丨Initial investigation of enantioselective synthesis of axially chiral arylindole.
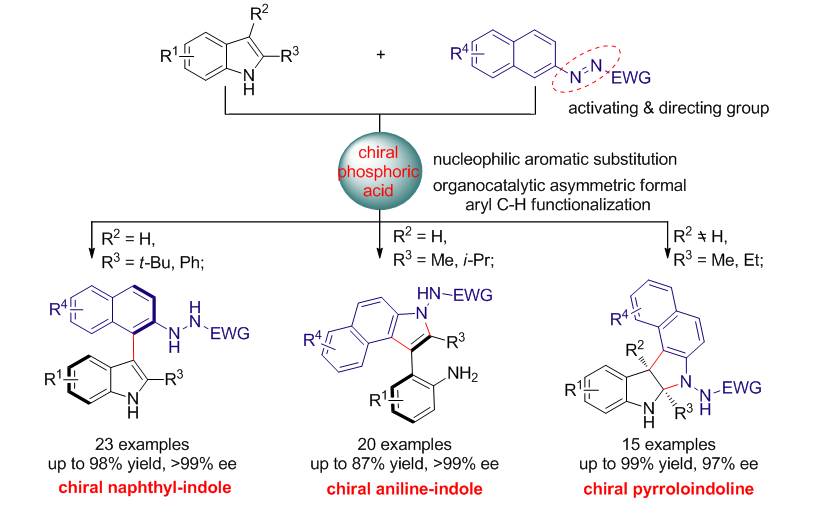
Figure 2 丨Current results via direct arylation of indoles.
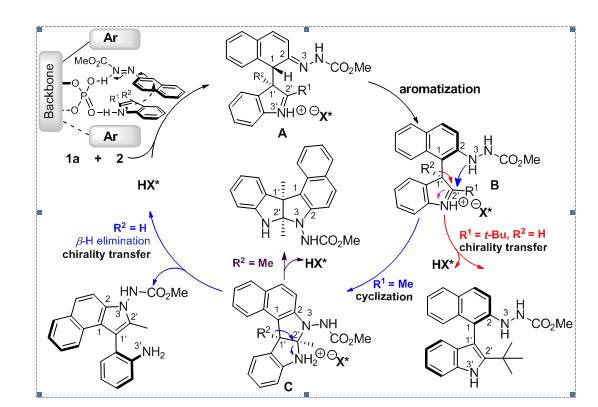
Figure 3丨Proposed reaction mechanism.
Tan’s group has discovered that the azo group can not only effectively activate an aromatic ring for nucleophilic attack, but also efficiently directs the formal nucleophilic aromatic substitution, which allows for the successful development of unprecedented organocatalytic enantioselective arylation of indoles.Researchers from Tan’s group anticipate that this strategy will foster the development of many other useful transformations and motivate new enthusiasm for organocatalytic asymmetric aryl functionalization.
The first author of this paper is Liangwen Qi, a postdoctoral student of SUSTech. Associate Professor Bin Tan is the only corresponding author. The research was funded by the National Natural Science Foundation of China, Shenzhen special funds for the development of biomedicine, the Internet, new energy and new material industries.
Associate Professor Tan gratefully acknowledges the support from SUSTech and President Shiyi Chen, for providing a world-class platform. Also, a special designed logo, named “Shiyi fund”, is dedicated to President Chen.

The paper links: http://www.nature.com/nchem/journal/vaop/ncurrent/full/nchem.2866.html
Proofread By
Photo By