Against the backdrop of rising global CO2 emissions, the development of low-energy and high-efficiency carbon capture technologies has become increasingly urgent. Traditional amine-based absorbents such as monoethanolamine (MEA) suffer from high regeneration energy consumption and low desorption efficiency. While piperazine (PZ) has a high absorption capacity, its low desorption efficiency limits its practical applications.

Professor Zuotai Zhang’s research team from the School of Environmental Science and Engineering at the Southern University of Science and Technology (SUSTech) has established an electronic structure-guided screening framework based on Density Functional Theory (DFT). Using this approach, they successfully developed highly efficient PZ derivative absorbents, achieving high CO2 absorption capacity, efficient desorption, and low-energy regeneration. This provides a new paradigm for the intelligent and precise screening and design of amine-based CO2 capture materials (Figure 1).
Their paper, titled “Electronic-Structure-Guided Screening of Piperazine Derivatives: Achieving Efficient CO2 Capture with High Cyclic Capacity and Low Energy Consumption,” has been published in Advanced Science.
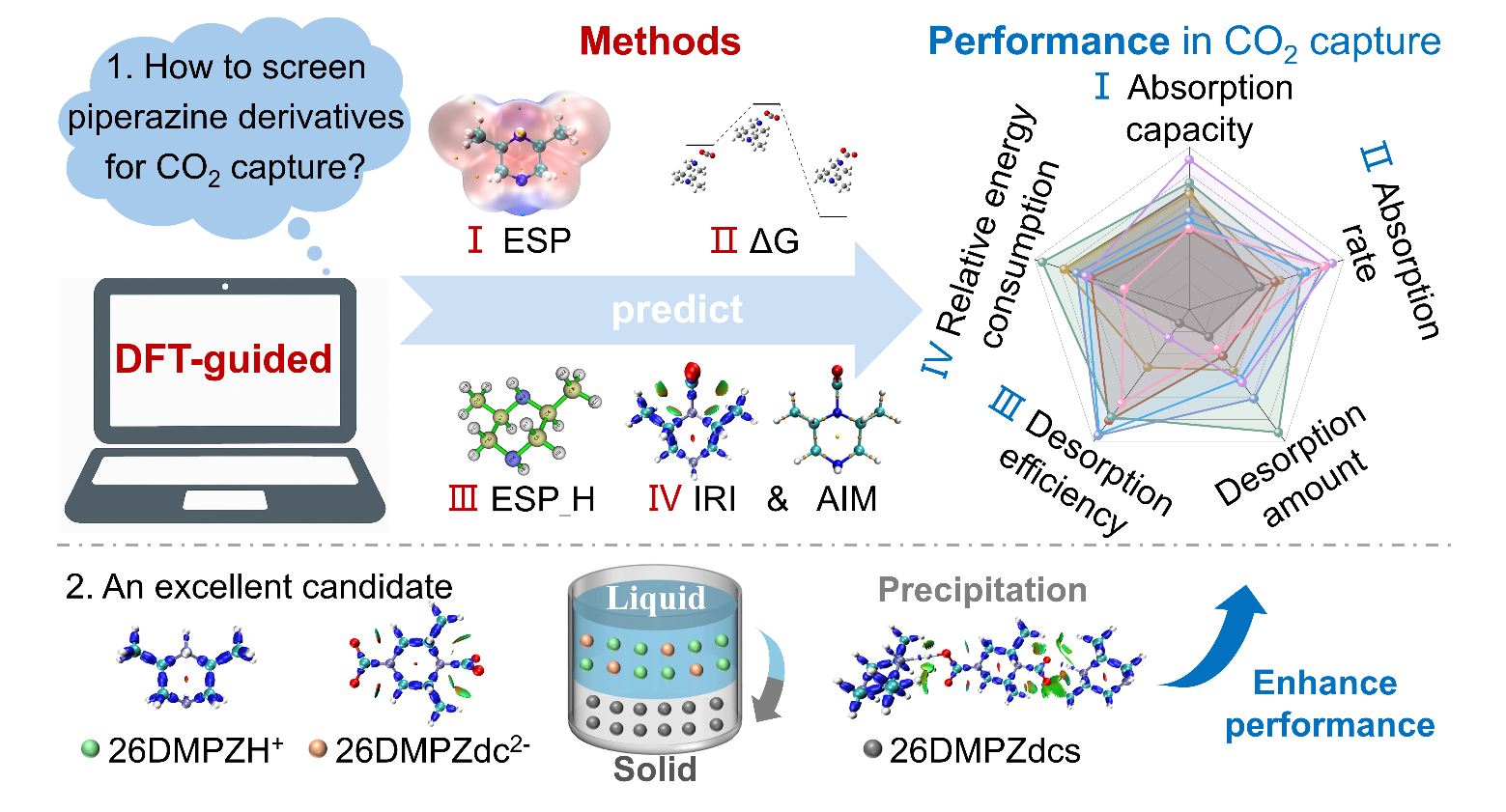
Figure 1. Construction of the electronic structure-guided screening framework based on DFT
The researchers proposed a DFT-driven molecular screening strategy that integrates multiple electronic descriptors, including molecular electrostatic potential (ESP), hydrogen nucleus electrostatic potential (ESP_H), reaction energy barrier (ΔG‡), and Gibbs free energy change (ΔG). This framework systematically predicted the CO2 absorption capacity, absorption rate, desorption efficiency, and energy consumption of eight PZ derivatives (Figure 2).
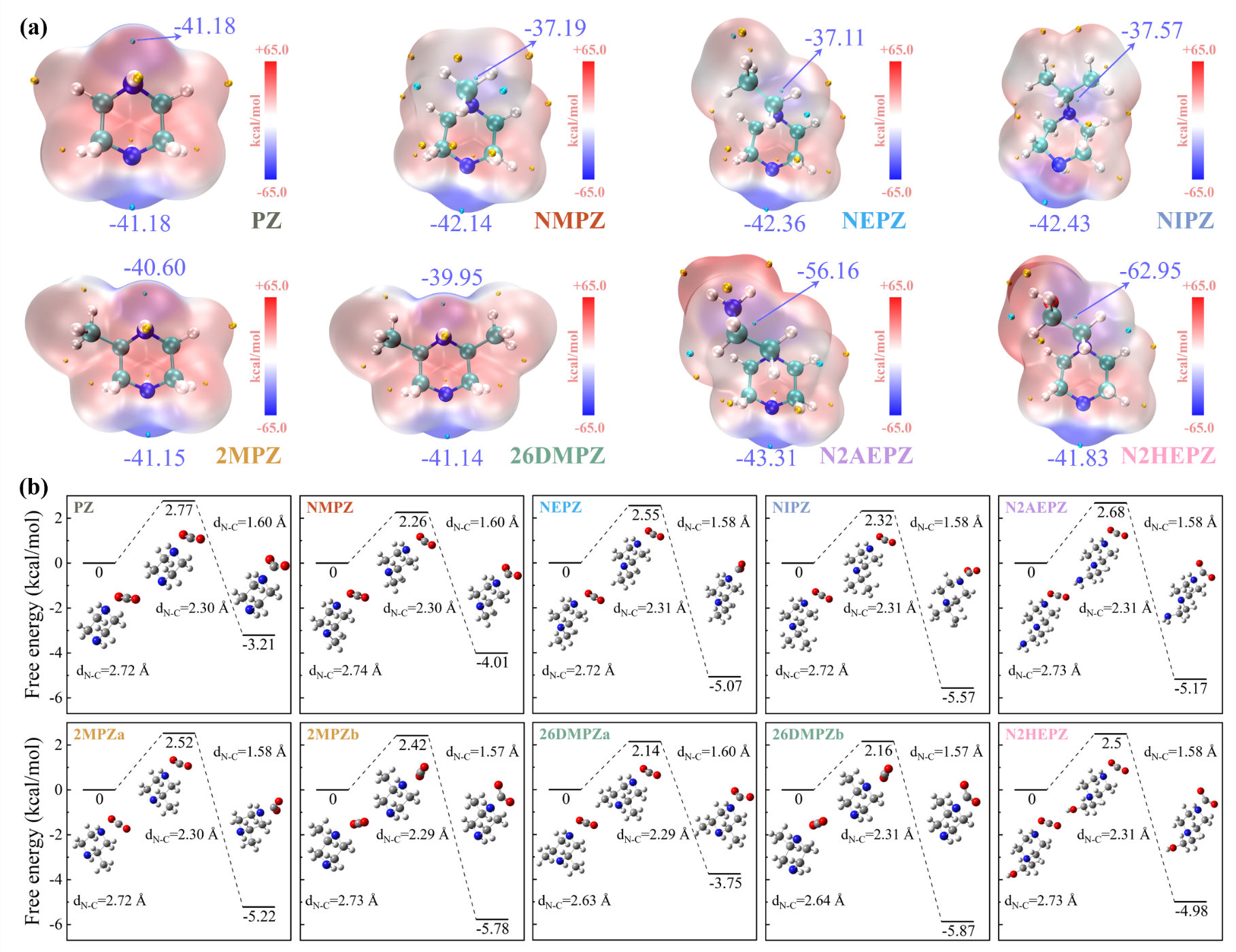
Figure 2. (a) ESP maps of PZ derivatives and (b) ΔG‡ and ΔG for the formation of zwitterion via the reaction between PZ derivatives and CO2
The team verified the theoretical predictions through systematic experimental tests (Figure 3), successfully confirming the reliability of their framework. They identified 2,6-dimethylpiperazine (26DMPZ) as the optimal candidate. This molecule exhibited excellent overall performance in experiments—achieving a CO2 absorption capacity of 0.94 mol/mol-amine, a desorption amount of 0.72 mol/mol-amine (about 80% higher than PZ), and 31% lower regeneration energy consumption compared to MEA. Importantly, 26DMPZ formed easily separable precipitates after absorbing CO2 in an aqueous solution. The “precipitate-driven phase separation” mechanism further enhanced absorption performance without the need for organic solvents, highlighting strong industrial application potential.
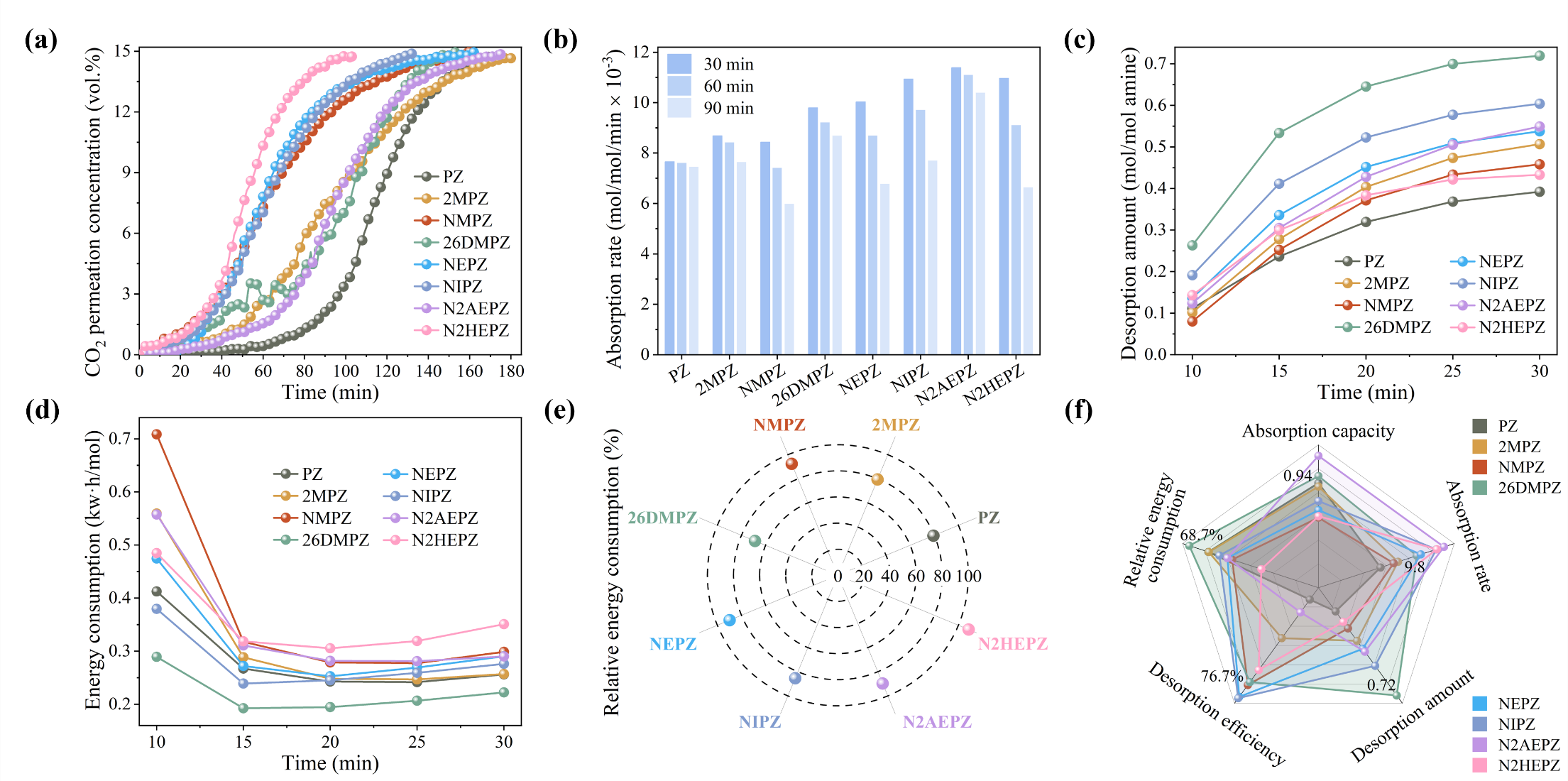
Figure 3. Experimental evaluation of CO2 absorption and desorption performance of PZ derivatives: (a) CO2 breakthrough concentration during the absorption process; (b) Average CO2 absorption rates at 30, 60, and 90 minutes; (c) Variation in CO2 desorption amount over 30 min; (d) Time-dependent energy consumption during desorption; (e) Relative energy consumption compared to 30 wt.% MEA; (f) Comprehensive performance comparison of each derivative
To further uncover the mechanism of performance enhancement, they used Nuclear Magnetic Resonance (NMR), High-Resolution Mass Spectrometry (HRMS), and Fourier-Transform Infrared (FTIR) spectroscopy, combined with solvation free energy (ΔGsolv) calculations (Figure 4a). The results revealed the precipitation pathway through which 26DMPZ and CO2 form an insoluble dicarbamate salt (Figure 4b). This precipitate decomposes completely at low temperatures (<120℃), significantly reducing regeneration energy consumption. Moreover, 26DMPZ maintained 98.4% of its initial cyclic capacity after ten rapid absorption-desorption cycles, demonstrating excellent cycling stability and industrial feasibility (Figure 4c).
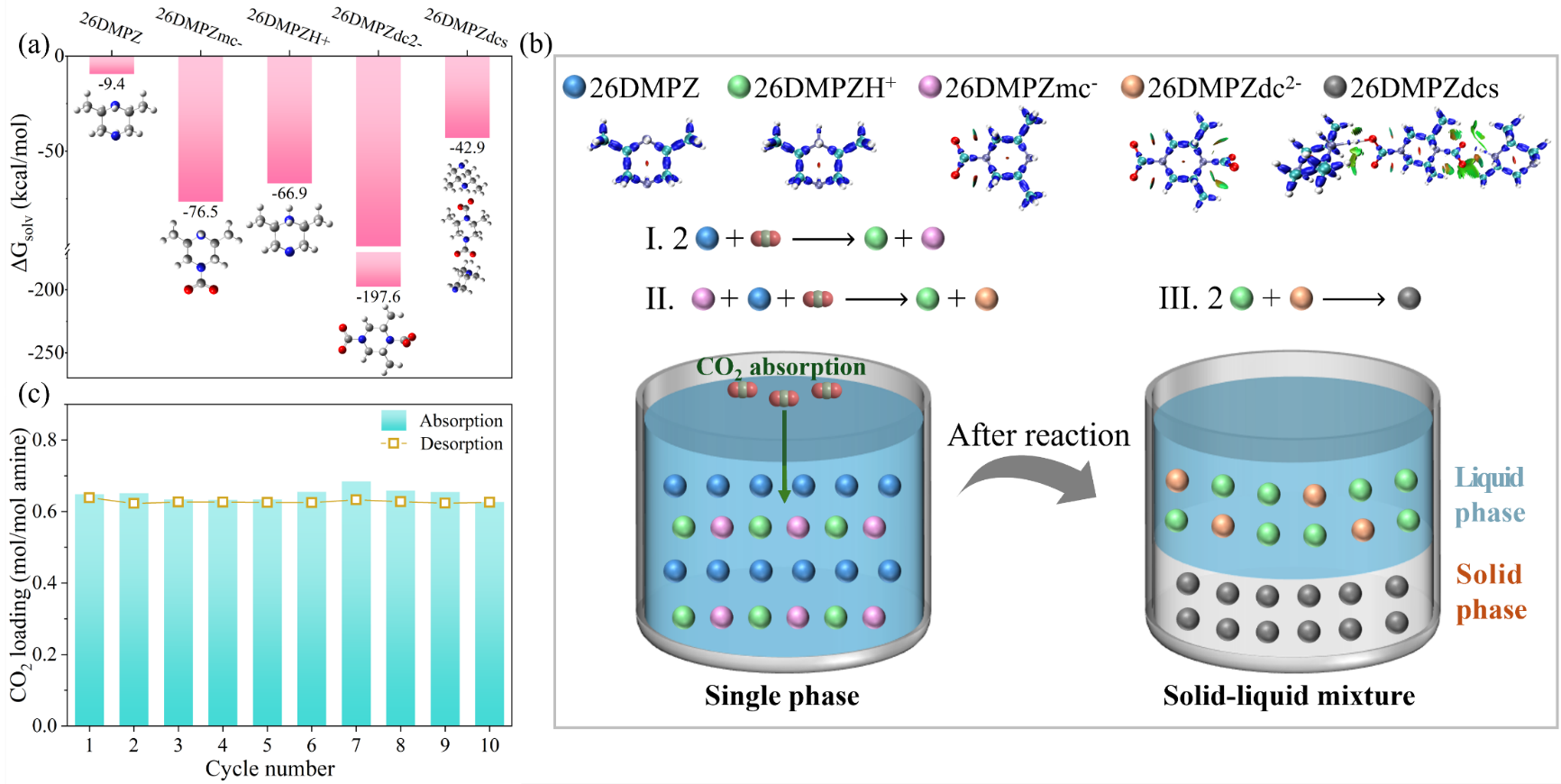
Figure 4. (a) ΔGsolv of key species involved in the reaction of CO2 absorption with 26DMPZ; (b) Proposed mechanism for CO2 absorption and precipitation pathway; (c) Cyclic performance of 26DMPZ under rapid absorption-desorption conditions
This study not only provides a reliable DFT-based screening framework that accelerates the development of high-performance amine absorbents but also showcases the tremendous potential of the “precipitate-driven phase separation” strategy in aqueous systems. Together, these findings provide both theoretical and technical support for the design of next-generation green, low-energy CO2 capture technologies.
Ph.D. student Feng Xie from the School of Environmental Science and Engineering is the first author of the paper. Professor Zuotai Zhang and Research Assistant Professor Xuehua Shen are the co-corresponding authors, with SUSTech serving as the first corresponding institution.
Paper link: https://doi.org/10.1002/advs.202513855
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yuwen ZENG
Photo BySchool of Environmental Science and Engineering