Very recently, Nucleic Acids Research (impact fator 9.11 ) published a paper contributed in part by Dr. WEI Zhiyi, the associate professor of Biology at SUSTech, demonstrating a sophisticated structural plasticity of a human tRNA synthetase for architectural reorganizations that are preferentially elicited in specific tissues.
This article reported the analysis of two closely related, internally deleted, splicing variants of homodimeric human tyrosyl-tRNA synthetase (TyrRS). The major contributors are Associate Professor Zhiyi Wei in Southern University of Science and Technology and Dr. Zhiwen Xu (Hong Kong University of Science and Technology), while the corresponding author is Professor Paul Schimmel (the Scripps Research Institute).
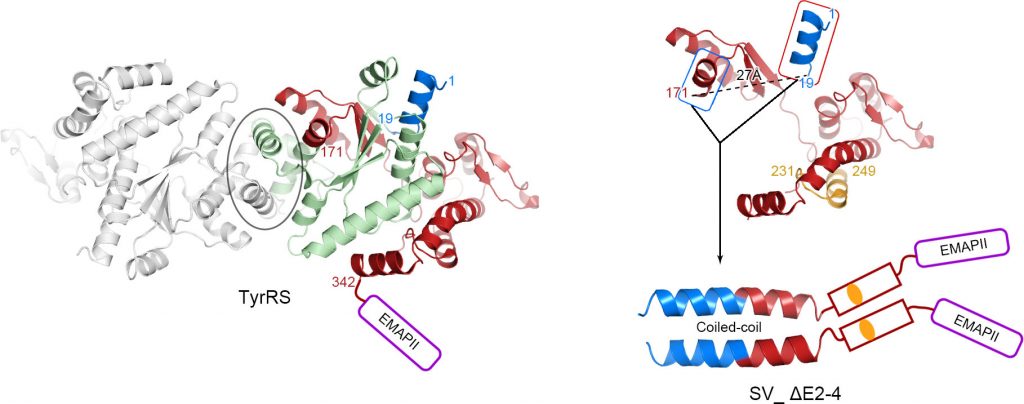
Aminoacyl tRNA synthetases (AARS) are ancient proteins that catalyze the ligation of amino acids to their cognate tRNAs. Their catalytic activities determine the genetic code and for that reason, they are essential components for protein synthesis in every living species. Recent studies revealed that human tRNA synthetases had evolved many alternative functions besides protein synthesis, including regulating angiogenesis, inflammatory responses, mTOR signaling as well as tumor growth. These functions are highly associated with over 200 alternative splice variants (SVs) found in human AARS family, most of which are catalytic nulls that engender new biology. The molecular modeling suggested that these internally deleted SVs may adopt large structural rearrangements. Despite of accumulating evidences showing their roles in regulate non-translational activities, very little is known about structures resulting from natural internal ablations of any proteins.
To understand the biological meanings of potential structural rearrangements, Dr. Zhiyi Wei and his collaborators from the international research team investigated the two closely related, internally deleted, SVs of TyrRS, which deleted exons 2–4 and 2–3, respectively. Both variants ablate a portion of the catalytic core and dimer-interface of the full length TyrRS. However, interestingly, each SV folds into a distinct structure. Biochemical assays together with nuclear magnetic resonance (NMR) analysis showed that one variant obtains a novel coiled-coil region by exon skipping thereby forming an alternative dimer interface. In contrast, the internal C-terminal splice site of the other variant lacking the coiled-coil region adopts a predominantly monomeric form. Unlike ubiquitous TyrRS, the neomorphs showed clear tissue preferences, which were differentially enriched in lymphocytes or lung, indicating for separate biological functions.
The study demonstrates a sophisticated structural plasticity of a human tRNA synthetase for architectural reorganizations that are preferentially elicited in specific tissues. It provides an start point for the study of new biological functions and potential disease medical utility of AARS splice isoforms in the future.
Link: https://nar.oxfordjournals.org/content/early/2016/01/14/nar.gkw002.full
Proofread By
Photo By