Health is the most precious wealth for human society, and the pursuit of health and happiness is the original driving force and ultimate goal of human’s all social activities. Today, global drug-synthesis manufacturing has stepped up in China. In 2012, the sales value of the industry for China’s chemical active pharmaceutical ingredients reached RMB 310 billion yuan, and since then China’s active pharmaceutical ingredients and export scale have been ranked No.1 in the world. However, with the extraordinary development of China’s pharmaceutical industrial capacity, problems such as the relatively-superficial level of the current pharmaceutical manufacturing technology, inefficiency of manufacturing, great emission of the “three wastes (i.e. gas waste, liquid waste and solid waste)” and the environmental hazards, have been growing sharply. Therefore, it is of great significance to explore a path towards the “green” synthesizing pharmaceutical technique.
The goal of the green synthesis is to realize highly selective and efficient chemical reactions, minimize by-products, achieve “zero emissions” and finally realize the “atom-economy reaction”. It is obvious that highly selective and efficient catalytic reactions are more in line with green synthesis’ basic requirements, compared to stoichiometric reactions. Professor Xumu Zhang has been long engaged in developing highly efficient and selective catalysis, and his team has been working on the development of chiral ligands and catalysts for years. Professor Zhang and his team have obtained lots of state of the art achievements, they have continuously been making efforts on innovation and devoting themselves to tackling the new problems in the technical filed.
Chemical reduction is the core technology of the pharmaceutical industry. Statistics show that chemical reduction is widely applied in the pharmaceutical synthesis, and it covers up to 16% of this kind of application (oxidizing reaction is only 4%). However, current utilization of stoichiometry of metal hydrides as reducing agents remains dominant in industrial productions, which cause a great amount of waste and heavy pollution. Meanwhile, with the market requirement and sales volume of chiral drugs rocketing each year (with a higher-than-15% growth rate) , the traditional chiral method of separating and synthesizing chiral drugs has wasted a lot of labor power and resources and caused great harm to the environment. In recent years, the chiral reduction technology mainly based on the asymmetric hydrogenation has been developed rapidly and effectively applied in many industrial productions of chiral drugs, significantly raising the synthetic efficiency of chiral drugs. The Nobel Prize in Chemistry 2001 was dividedly awarded to American scientist William S. Knowles (prize share 1/3) and Japanese scientist Ryoji Noyori (prize share 1/3) for their excellent work on catalytic asymmetric hydrogenation, fully recognizing catalytic asymmetric hydrogenation’s positive contribution to human society.
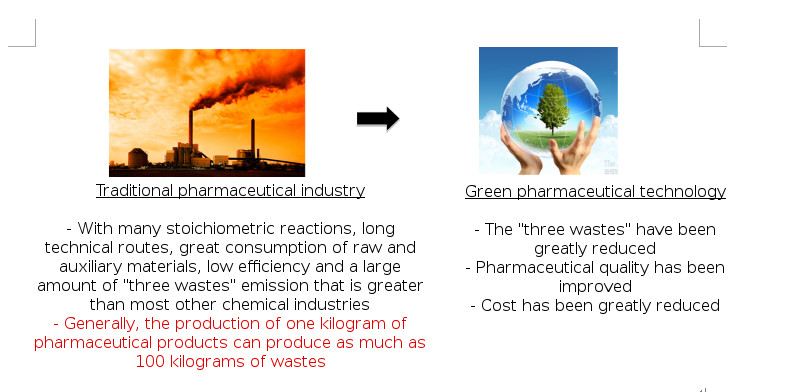
Comparison diagram of the effects from the traditional pharmaceutical industry and “green” pharmaceutical technology
The core of the research on the catalytic hydrogenation reaction is to develop efficient, high-selectivity hydrogenation catalysts and apply the catalysts into the “green”, economic and efficient synthesizing process of the important, functional substances required by humans. Although thousands of chiral catalysts have been developed, most turnover numbers (abbreviated TON) (referring to the number of moles of substrate that a mole of catalyst can convert before becoming inactivated. It is one of the indicators reflecting the catalyst’s reaction capability.) and turnover frequencies (abbreviated TOF) (the frequency of the substrate’s conversion with one mole of catalyst. It is the indicator reflecting the reactivity.) are still low, and the efficient chiral hydrogenation catalysts that satisfy the industrial requirements are still in a relatively small amount. The structure of the catalyst determines its built-in function and activity. The basic structural features, electronic effects, steric effects of transition metals and ligands directly affect the activity and selectivity of the catalysts. As there is no universal hydrogenation catalyst, the selection of transition metals and the design of new-type ligands are still the key for developing new-type catalytic hydrogenation system and the core for further broadening the catalytic hydrogenation’s functions and activity. In addition to developing the new-type hydrogenation catalysts that have various structures and different electrical properties and steric hindrances, developing the new modes and new concepts of hydrogenation is of the same importance in solving different hydrogenation problems. The development of the new modes and new concepts for the activation of hydrogenation catalysts and substrates is the important part of developing the frontier researches on the efficient, high-selectivity reduction.
For many years, Professor Xumu Zhang and his team have been working on the development of the chiral ligands and catalysts. They have developed many types of novel-structure, superior-property chiral phosphine ligands and catalysts and applied them into the asymmetric hydrogenation, hydroformylation, enyne cycloisomerization and other asymmetric catalytic reactions. Professor Zhang and his team have obtained many advanced results especially in the field of asymmetric catalytic reactions. According to the search results obtained from the SciFinder® database, Professor Xumu Zhang has kept a leading place in the world on the research of both chiral phosphine ligands and asymmetric hydrogenation.
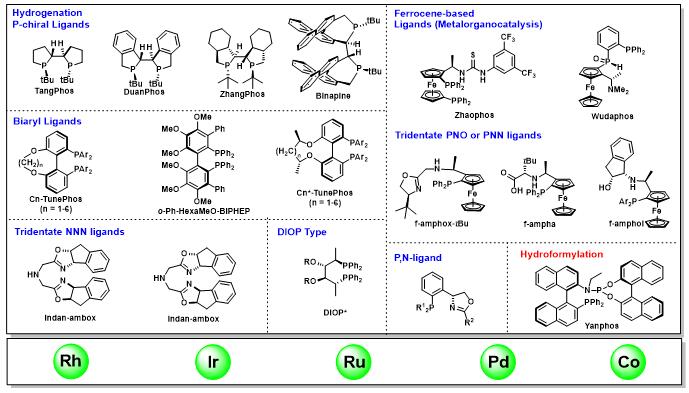
Chiral toolbox of Xumu Zhang’s team
Additionally, these projects and results have been successfully applied in industrialization for many years. For instance, the application of the asymmetric hydrogenation is in the lead in major pharmaceutical synthesis industrialization application fields in China and worldwide; the industrialized application of the new green synthesis technology for major drugs, such as the intermediates of Duloxetine and Sitagliptin, has a large scale, causes low pollution and can bring in extremely high yields (- its production value has reached hundreds of millions of yuan in the recent few years). Several major active pharmaceutical ingredients have been awarded with the DMF (Drug Master File) numbers by US FDA (US Food and Drug Administration).
The advanced synthetic process of the Duloxetine intermediates is another example. Duloxetine is a selective serotonin (5-HT) –norepinephrine (NE) reuptake inhibitor, which is used mainly in the treatment against major depression disorder. Duloxetine is Eli Lilly and Company’s top-selling drug, with a sales volume over 5 billion dollars and hundreds of tons of consumed active pharmaceutical ingredients annually.


For China’s traditional process: the original process routes of the key intermediates have to be divided; the conditions of the demethylation reaction in the last step are harsh; total yield is low; a large amount of waste is produced; and relevant pollution and meaningless consumption are heavy.
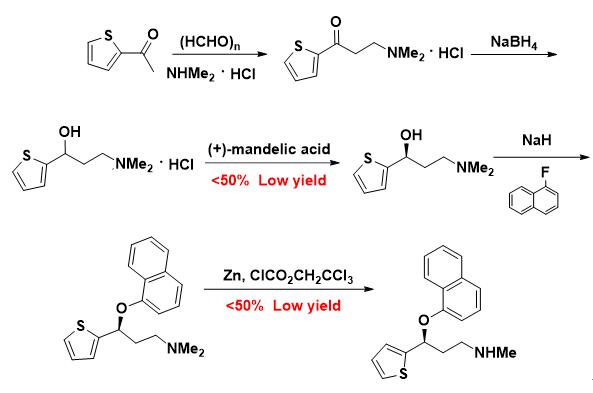
China’s traditional process of Duloxetine
As there are chiral Aryl-Methanol structures in Duloxetine’s molecular structure, Professor Xumu Zhang and his team have successfully developed the advanced green synthetic process by realizing the above process with the asymmetric hydrogenation of aryl ketone: use the efficient catalytic asymmetric hydrogenation as the key step, with only three steps and without demethylation. No isomer waste is produced therefrom, reducing the cost by 74%.
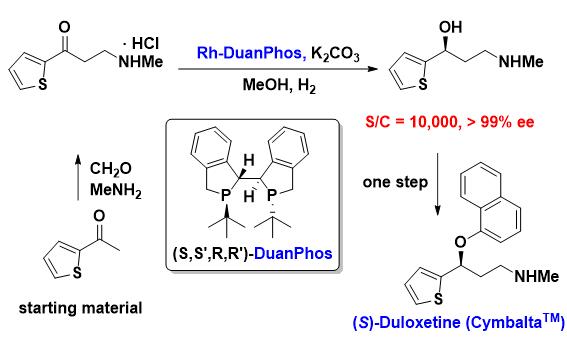
New process of Professor Xumu Zhang’s team
Overcoming pharmaceutical obstacles to benefit mankind
Professor Xumu Zhang and his team have made great efforts on innovation and overcoming arising technical problems and keep explorating and innovating new process routes for antibacterial drugs, based on the advanced synthetic techniques obtained at the earlier stage. Taking the synthetic process of the carbapenems as an example, Professor Xumu Zhang and his team have been working on exploration and innovation in order to continue making contributions to the disease treatments of human beings.
Carbapenems (also known as antibiotic carbapenem) has been known by the public since 1980s and is an atypical Beta-lactam antibiotic (β-lactams). It has the broadest antibacterial spectrum and the strongest antibacterial activity among the antibacterial drugs that have been developed up to now. It features broad spectrum, strong activity, and low incidence rate of the resistance to carbapenems. Globally, 7 carbapenems drugs have been marketed. They are (in the order of the years of their release): imipenem, panipenem, meropenem, faropenem, ertapenem, biapenem and doripenem. According to the analysis of the industry experts, the drug-resistance problem of cephalosporins and other drugs that have been on the market for many years is the main cause of the carbapenems’ rapid emergence in such a short time. As 4-AA (4-Acetoxy-2-azetidinone) is the main raw material for the production of all active pharmaceutical ingredients for antibiotic carbapenem (i.e. Carbapenems), the efficient, high-selectivity acquisition of the chiral intermediate is the most important step for the production of carbapenems. The structural molecular formula of 4-Acetoxy-2-azetidinone is shown in the following figure.
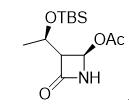
4-Acetoxy-2-azetidinone
Currently, Professor Xumu Zhang and his team have stepped out in the further exploration and research for the synthetic process of the carbapenems intermediates and have obtained efficient, high-selectivity green synthetic process. They hope to obtain the chiral intermediates from a more mature green synthesis process with unremitting efforts.
At the same time, Professor Xumu Zhang and his team have reached a cooperation agreement with several large pharmaceutical enterprises in Shenzhen and will continue to focus on the conquest of clopidogrel, montelukast, benazepril, 4-AA (a carbapenem’s key intermediate) and other kinds of widely-used drugs. They will surely contribute more to the production of the pharmaceuticals that will benefit mankind.
Proofread By
Photo By