Southern University of Science and Technology (SUSTech) has started the new year with the research group of Department of Chemistry Professor Zhi Zewei published in two important academic journals. One of the research papers was selected as the Supplementary Cover of J. Am. Chem. Soc. (JACS), while the earlier article was published in Angew. Chem Int. Ed. Postdoctoral researcher Dr. Li Wei was the first author, while Nanyang Technological University (NTU), Jilin University, Cornell University and Huazhong University of Technology were the main communication units.
The paper published in Angew. Chem. Int. Ed was titled “High-Pressure Band-Gap Engineering in Lead-Free Cs2AgBiBr6 Double Perovskite.” They studied the structure and properties of lead-free double-calcium titanium Cs2AgBiBr6 under high pressure. Double-perovskite has become a center for perovskite research due to its advantages of non-toxicity and stability. Its application in optoelectronic devices is currently limited due to the large band gap (2.2 eV) of Cs2AgBiBr6, so its controllable regulation is a major point of study for researchers.
In this work, diamond-based anvil (DAC) was used to characterize the structure and properties of Cs2AgBiBr6 with pressure by in-situ Raman spectroscopy, UV-visible absorption spectroscopy and X-ray diffraction under high pressure. It was found that Cs2AgBiBr6 will change from face-centered cubic to tetragonal at 3 GPa pressure, resulting in tilting distortion of AgBr6 and BiBr6 regular octahedron in the crystal, reducing the coincidence of electron orbitals, and increasing the band gap of Cs2AgBiBr6 in the tetragonal phase. The pressure was continuously increased to 6.5 GPa, and the Cs2AgBiBr6 crystal showed amorphization tendency, and the band gap gradually decreased to 1.7 eV.
After the pressure is released to normal pressure, the amorphous portion remaining in the material retains the properties under high pressure, and the band gap of the lead-free perovskite Cs2AgBiBr6 under normal pressure is reduced by 8.2%. This work has important research significance for exploring the relationship between the structure and properties of double perovskite and the controllable regulation of properties under high pressure.
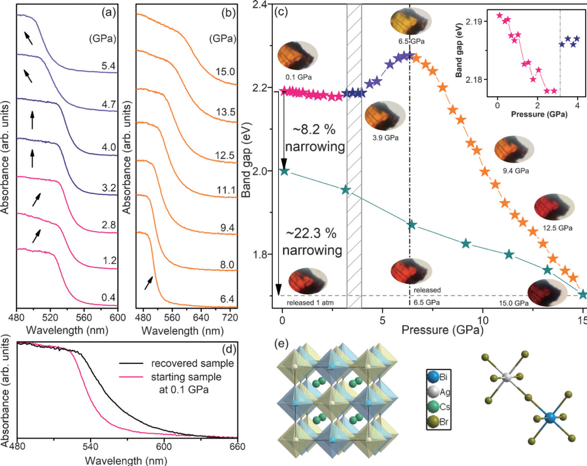
Figure 1. Change in band gap under high pressure of Cs2AgBiBr6.
The paper published in JACS was titled “Pressure-Induced Phase Engineering of Gold Nanostructures.” In nature, precious metal gold (Au) blocks can only exist in their thermodynamically stable structural face-centered cubic (fcc) phase. Only at the nanometer scale, using wet chemical synthesis methods, one can obtain gold nanomaterials with close-packed hexagonal hcp-4H structure with unique optical properties. Although the gold in the 4H phase is induced to become an fcc structure in solution by ligand exchange or epitaxial growth of noble metal, more structural information is obtained. However, the specific structural properties and phase transition process remain uncertain. In this work, the diamond nano-anode (DAC) technology was used to study the 4H phase gold nanomaterials, and the structure and phase transformation process were explored to achieve the purpose of high-pressure precious metal phase engineering.
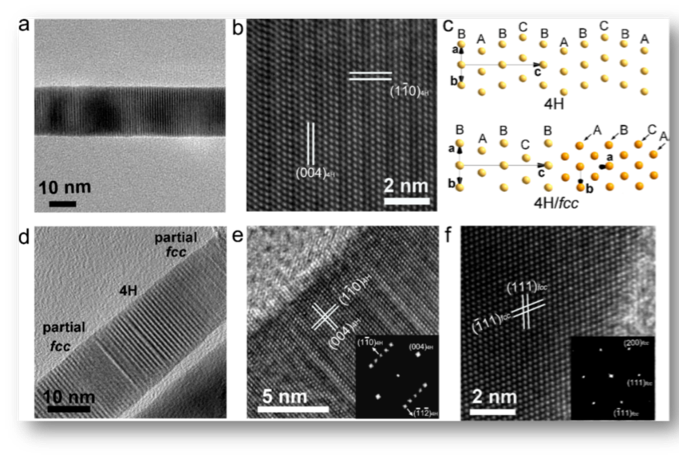
Figure 2. Modulation of nanostructured Au structures by high pressure.
High-pressure X-ray diffraction showed that the pressure was between 1.2 and 26.1 GPa, and the 4H structure of gold gradually changed to the fcc phase. At the same time, the irreversibility of the process makes high-pressure phase engineering of precious metals possible. That is, by controlling the highest pressure, gold nanomaterials having different 4H/fcc phase contents are obtained. At the same time, the 4H/fcc gold nanorods with alternating 4H and fcc phase structure are more prone to high-pressure phase transition than the pure 4H phase gold nanobelts. This is mainly due to the phase-to-nuclear sites provided by a large number of phase boundaries in the 4H/fcc multiphase gold nanorods, which can promote the phase transition process of 4H-fcc.
In addition, the atomic-scale Au phase transition path was first observed by the combination of high-resolution transmission electron microscopy and density functional theory (DFT) calculations. The phase transition mechanism of Au from 4H-fcc was found to be the leveling of the (-112) 4H crystal plane, accompanied by a change in the direction of close packing. This is completely different from the phase transition mechanism of the metal high-pressure hcp-fcc phase observed in the past. This work not only provides new insights into the stability and phase transition of gold nanostructures but also provides a new strategy for using pressure to regulate the crystalline phase content of noble metal nanomaterials. This strategy can be used to study the crystallization of crystals based on issues such as phase, enhanced Raman scattering, waveguides, photothermotherapy, sensing, and clean energy. The paper was selected as the Supplementary Cover of the issue.
The above research has received strong support from the National Natural Science Foundation of China, the Shenzhen Science and Technology Commission, the start-up funds of the South University of Science and Technology and the Principal Fund.

Figure 3. The JACS cover (Supplementary Cover).
Related paper links:
https://onlinelibrary.wiley.com/doi/full/10.1002/anie.201708684
Proofread ByXia Yingying
Photo ByDepartment of Chemistry