Pulmonary arterial hypertension is a condition where the blood pressure in the arteries in the lung is higher than it should be. It is a serious problem and at this point in time, the cause is unknown. With various treatment options requiring injections into the skin, much like a diabetic might take insulin, there has been significant research into the best way to properly synthesize the more complex components of these drugs.
Researchers from Southern University of Science and Technology (SUSTech) have found a new method for synthesizing a critical component of the drug Treprostinil. The research team, led by Professor Zhang Xumu of the Department of Chemistry, published their paper in renowned international journal Nature Communications (IF = 12.353) with the title, “Enantioselective Rhodium-Catalyzed Cycloisomerization of 1,6-Allenynes to access 5/6-Fused Bicycle[4.3.0]nonadienes.”
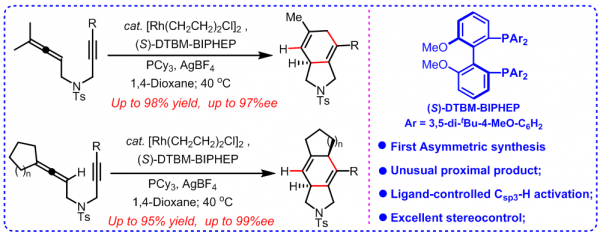
The research team aimed to find a way to synthesize an asymmetric version of complex polycyclic skeletons from simple linear substrates. In other words, these organic chemical compounds have complicated structures with multiple closed rings that the research team wants to manufacture from simple chemicals that have a linear relationship between the amount of product created and the amount of chemical used.
In the past, a wide range of metals have been used as catalysts, which reduces the energy required for a chemical reaction to take place. Rhodium, gold, platinum, ruthenium, palladium, cobalt, titanium, silver, gallium and molybdenum have all been used as catalysts in various experiments in the past, with rhodium providing the most interest to researchers.
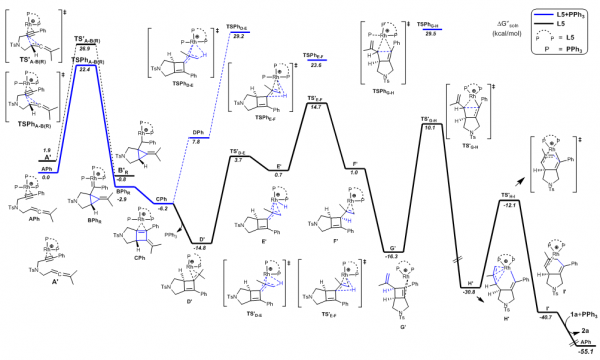
The research team focused on synthesizing asymmetric version transition-metal-catalyzed cyclizations. They were able to generate the intended organic chemical compound, called an enatioselective rhodium(I)-catalyzed cycloisomerization of 1,6-allenynes. This was achieved through what they believe is an unusual cyclization (or carbon ring), that features 5 atoms that originally broke outside the ring with a double bond. The ring is attached to a molecule of the same content, but different layout. This structure allows for the synthesis in good yield and excellent enantioselectivity, or how much of the compound is created during the chemical reaction.
This research is critical to the development of different medicines, particularly for widening the arteries in the lungs. However, it has a greater academic significance, rather than commercial or industrial significance, as far as the research team is concerned.
Professor Zhang Xumu worked with SUSTech Associate Professor Oscar Chung and Wuhan University Associate Professor Lv Hui from the School of Chemistry and Molecular Sciences. Professor Zhang Xumu said that the research reflected a typical interdisciplinary approach and that the team had long been committed to highly selective and efficient catalytic systems. The results prove that there are many different potential pathways through various chemical calculations worthy of exploration.
Professor Zhang Xumu and his team plan to further develop their selective catalytic system based on the results of this study. They aim to overcome the limitations of the current methods and expand on them to account for more challenging and application-oriented organic chemical compounds that could be synthesized in one step.
The project received funding from the National Natural Science Foundation of China (NSFC), SUSTech, Central South University, the Nature Science Foundation of Hunan Province, the Natural Science Foundation of Hubei Province, the Shenzhen Nobel Prize Scientists Laboratory Project and Changsha Science and Technology Projects.
Original article can be found at https://www.nature.com/articles/s41467-019-08900-z
Proofread By
Photo ByDepartment of Chemistry