Exploring low-cost and efficient electrocatalysts for hydrogen evolution reaction (HER) from water splitting is important for hydrogen production. Hydrogen is a renewable and clean fuel that has great potential for solving the energy crisis. At present, the precious metal Pt is still the most active candidate catalyst with negligible overpotential for HER. However, the high cost and limited reserves of Pt hinder the commercialization of Pt-based catalysts. Thus, lowering the Pt loading of catalysts without sacrificing HER catalytic activity is an urgent goal. Single-atom catalysts (SACs) with isolated catalytic sites provide an alternative way to solve the above problems. However, the harsh preparation conditions (calcination or H2 reduction) and excess precursors usually result in agglomeration, hindering the stability of SACs.

With this in mind, the Cryo-Electron Microscopy Center (Cryo-EM Center) and the Pico Electron Microscope Center at Southern University of Science and Technology (SUSTech) has provided the opportunity for researchers like Professor Gu Meng of the Department of Materials Science and Engineering to develop new energy materials to improve the increasingly serious energy shortage faced by the world. Professor Gu Meng led his research team to recently publish a papers in Energy & Environmental Science (IF > 30) entitled “Highly stable single Pt atomic sites anchored on aniline-stacked graphene for hydrogen evolution reaction.” It was included in the hot articles for 2018 and 2019.
With hydrogen being an important electrocatalyst for future energy utilization and conversion, given its low cost and hydrogen efficiency, it is important to understand how to best produce it from water cracking. At this point in time, platinum (Pt) is the most frequently used metal for this purpose, but its high cost and limited reserves means that alternative sources must be considered. Recent research has suggested the application of monoatom catalysts with independent catalytic sites, but the harsh preparation conditions and excessive precursors have prevented the commercial uptake of this approach.
Professor Gu Meng’s team opted to disperse Pt atom sites anchored on aniline-stacked graphene, in order to make the best use of Pt’s catalytic performance. The experiment showed that the volume of Pt could be greatly reduced, while significantly improving the quality and activity of its catalytic performance. Results also demonstrated that the performance of commercial Pt-C catalysts can be achieved when the Pt level is reduced to 2% of the current rate. Aniline anchoring was simultaneously shown to prevent aggregation of atoms, improving stability.
This work opens up a new way for the development of monoatomic catalysts and promotes new understandings of catalytic performance of monoatomic catalysts.
Co-first authors were Ye Shunghua, Luo Feiyan, Zhang Qianling, Zhang Pingyu and Xu Tingting – all from Shenzhen University. Correspondent authors are Professor Gu Meng from Southern University of Science and Technology, Liu Jianhong from Shenzhen University, and Sun Xueliang from the University of Western Ontario.
They also received strong support from Professor He Jiaqing, Director of the SUSTech Pico Electron Microscope Center and Professor Wang Peiyi, Director of the SUSTech Cryo-EM Center.
They received funding from the National Natural Science Foundation of China (NSFC), the Key Project of Natural Science Foundation of Guangdong, the Shenzhen Basic Research Layout Project, and the Major Programs for Science and Technology Development of Shenzhen.
Original paper – https://pubs.rsc.org/en/content/articlelanding/2019/ee/c8ee02888e#!Divabstract
Further to this, Professor Gu Meng’s group has been working with Professor Dai Hongjie of Stanford University on the development of safe rechargeable battery systems. While they have been widely used in recent years, battery fires and explosions are a common cause for concern. Combustible organic electrolytes present a risk of fire or explosion, so researchers have been investigating other electrolytes that are inherently non-flammable.
The most popular candidate for this has been room-temperature ionic liquids (ILs), due to their non-flammable properties. The research teams of Gu Meng and Dai Hongjie developed a chloroaluminate ionic liquid electrolyte based on aluminum chloride/1-methyl-3-ethylimidazole/sodium chloride ionic liquid. The rechargeable sodium metal battery based on this ionic liquid electrolyte has good performance, the battery voltage is up to ~ 4v, the coulombic efficiency is up to 99.9%, and the energy and power density are ~ 420 Whkg-1 and ~ 1766 Wkg-1 respectively.
The IL electrolyte maintained more than 90% of its capacity after 700 cycles, proving itself to be a promising candidate in preparing sodium batteries, and offers potential for other rechargeable batteries such at lithium or potassium based batteries.
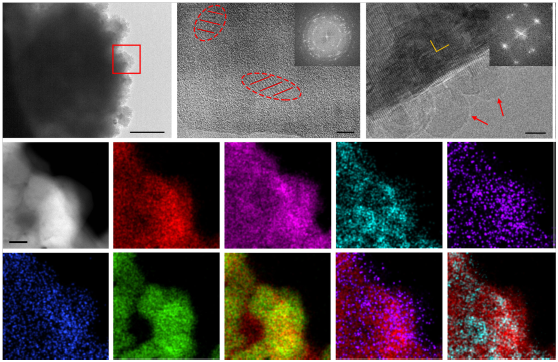
Professor Gu Meng’s research team used cryo-electron microscopy to analyze the solid electrolyte interphase (SEI) film produced in the battery system composed of ionic liquid electrolyte. The experimental results show that the SEI film obtained by the reaction in the ionic liquid electrolyte is composed of various components, and its main components are NaCl, Al2O3 and NaF. The results provide a vital guide for the future design of practical sodium metal batteries with high safety and high energy density from the perspective of electrolyte and SEI film.
The results of this research were published in Nature Communications earlier this year, in a paper entitled “A safe and non-flammable sodium metal battery based on an ionic liquid electrolyte.
This work was completed by the cooperation between Professor Gu Meng and Stanford University Professor Dai Hongjie, at the Stanford Nano Shared Facilities (SNSF).
The research received funding from the Stanford Bits and Watts Program, the National Science Foundation and gift funds, among other sources.
Original paper – https://www.nature.com/articles/s41467-019-11102-2
Proofread By
Photo ByDepartment of Materials Science and Engineering