Ceramides are the central molecules in sphingolipid metabolism in eukaryotes and also serve as crucial signaling molecules that regulate various cellular processes such as apoptosis, aging, proliferation, and differentiation. Abnormal accumulation of ceramides is a hallmark of many human metabolic diseases, including obesity, diabetes, and cardiovascular diseases. Ceramide synthase (CerS) catalyzes the de novo synthesis of ceramides in the endoplasmic reticulum by condensing the sphingoid base and fatty acyl-CoA. CerS is considered a potential therapeutic target for diseases such as obesity, insulin resistance, cardiovascular diseases, non-alcoholic fatty liver disease, and cancers.
More than twenty years ago, CerS was first discovered in the yeast Saccharomyces cerevisiae, where two redundant genes encoding proteins called Lag1 and Lac1 were found to be involved in acyl-CoA-dependent ceramide synthesis. Further studies identified a regulatory subunit called Lip1 that can form complexes with Lag1 or Lac1. However, the specific molecular mechanism by which the Lip1 regulatory subunit functions in ceramide synthesis remains unclear. The lack of structural information about CerS has greatly limited our understanding of its function.
The research team led by Associate Professor Xin Gong from the School of Life Sciences at the Southern University of Science and Technology (SUSTech) has recently reported the first atomic-resolution cryo-electron microscopy structure of eukaryotic ceramide synthase and revealed the molecular mechanism underlying its function.
Their research work, entitled “Structure and mechanism of a eukaryotic ceramide synthase complex”, has been published in The EMBO Journal.
In this study, the researchers obtained the recombinant expression and purification of the yeast ceramide synthase Lac1-Lip1 complex through in vitro methods and established in vitro biochemical assays to measure its catalytic activity. Subsequently, they used single-particle cryo-electron microscopy to determine the high-resolution three-dimensional structure of the complex bound to the substrate C26-CoA (Figure 1). The resolved structure revealed that the yeast ceramide synthase forms a complex in a 2:2 ratio through Lip1 dimerization on the basis of the Lac1-Lip1 heterodimers. In each Lac1-Lip1 protomer, a C26-CoA molecule was observed within the Lac1 subunit. The extensive interactions between Lip1 and Lac1 are crucial for maintaining the binding pocket of the C26-CoA substrate, explaining the activating role of the regulatory subunit Lip1 in the complex.
Based on the resolved structure and structure-guided mutagenesis studies, the researchers proposed a catalytic model for ceramide synthesis by yeast ceramide synthase (Figure 2). In this model, the C26-CoA substrate enters the reaction chamber of the Lac1-Lip1 dimer from the cytoplasmic side of the membrane, with the CoA portion of C26-CoA located in the hydrophilic chamber of Lac1, while the acyl chain of C26-CoA binds to the hydrophobic tunnel formed by Lac1 and Lip1. Another substrate, sphinganine, may enter the reaction chamber through a lateral opening on Lac1.
Although many questions still need further investigation, the findings of this study are of great significance for understanding the biological function of eukaryotic ceramide synthases. They may facilitate the rational design of ceramide synthase modulators for the treatment of metabolic diseases and cancer in the future.
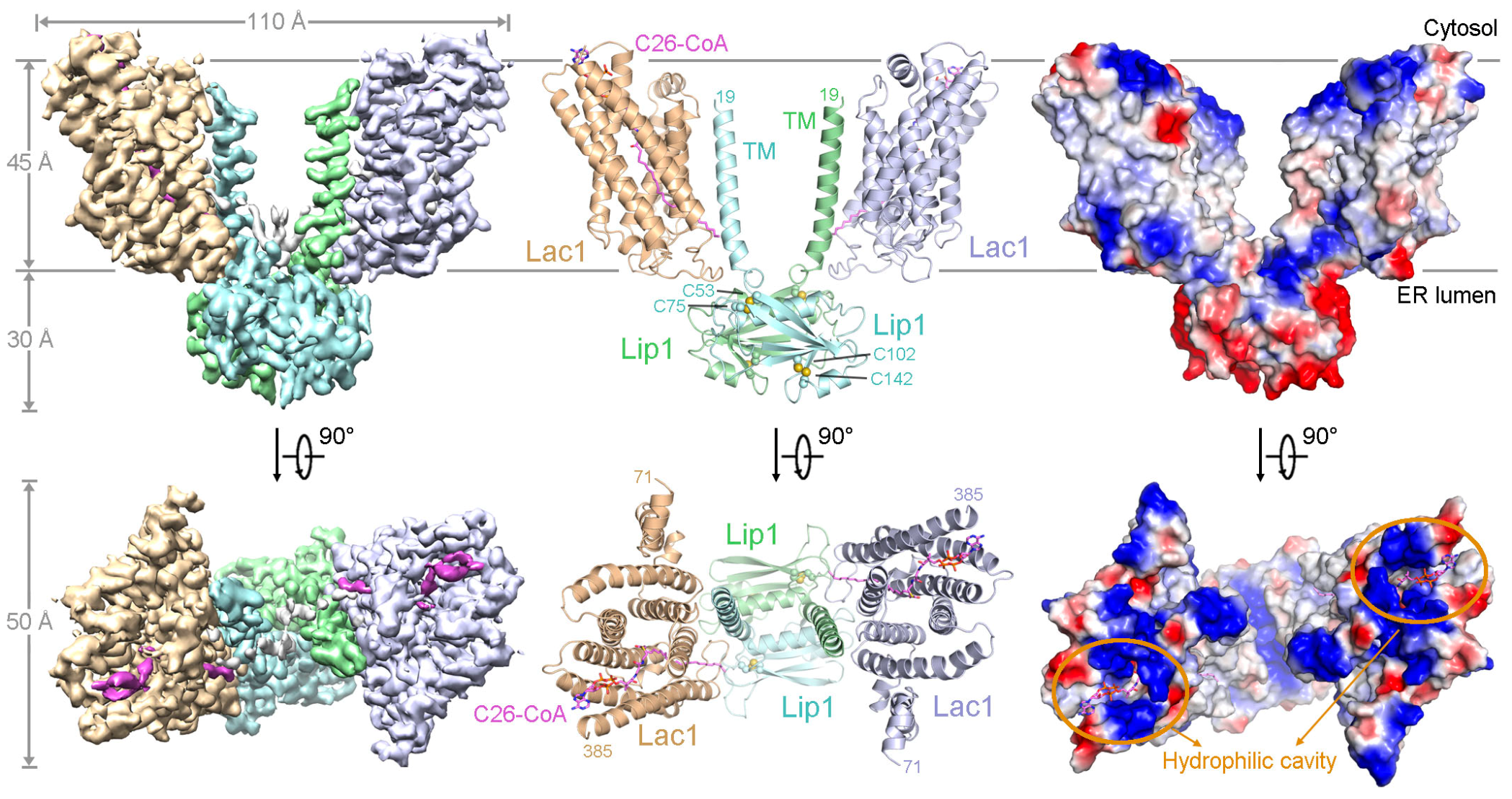
Figure 1. Overall structure of the yeast Lac1-Lip1 complex bound to C26-CoA
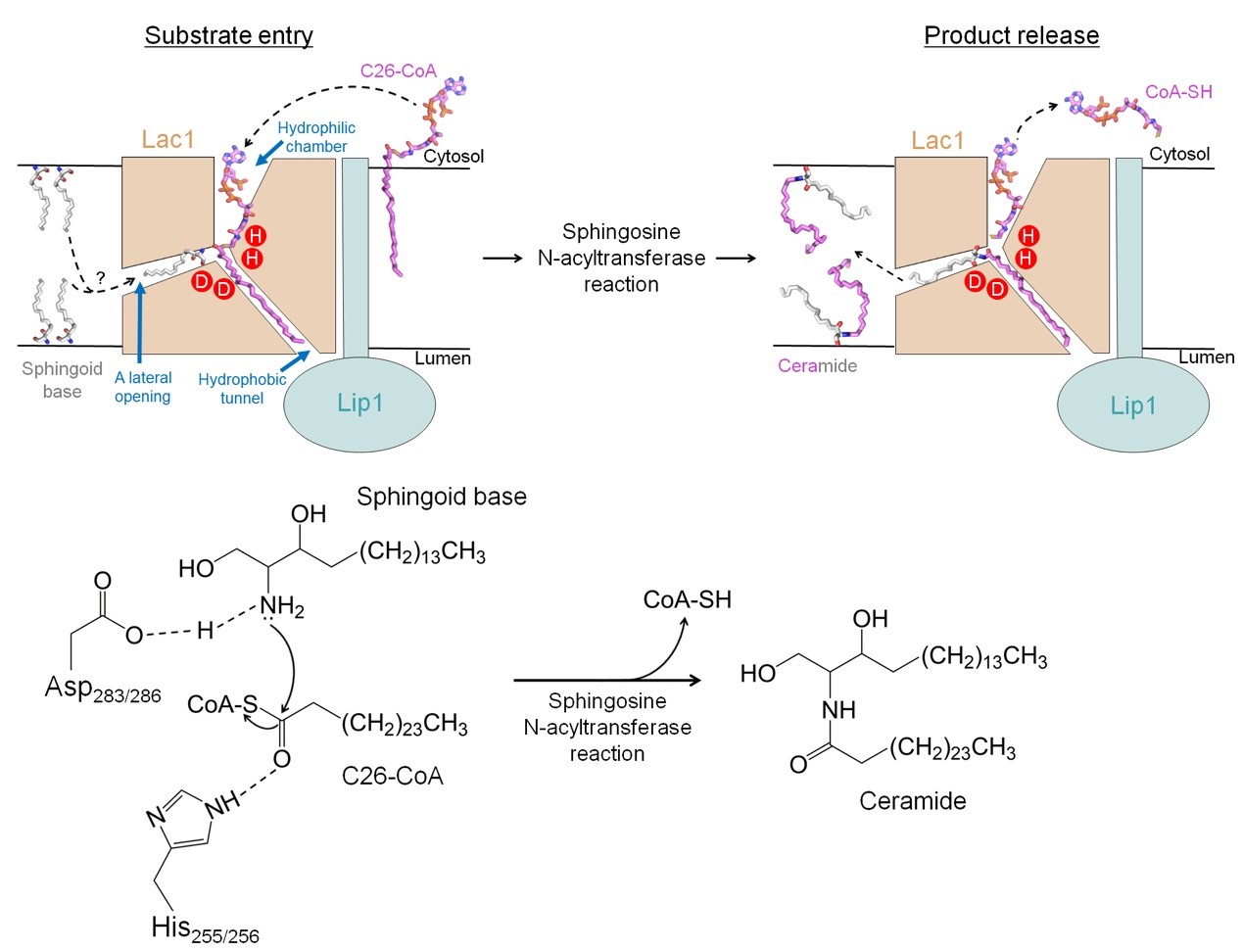
Figure 2. Proposed working mechanism of yeast CerS
Research Associate Professor Tian Xie and Ph.D. student Qi Fang from the School of Life Sciences at SUSTech are the co-first authors of this paper. Associate Professor Xin Gong is the corresponding author.
This work was supported by the National Natural Science Foundation of China (NSFC), Guangdong Basic and Applied Basic Research Foundation, and the Shenzhen Science and Technology Program. The cryo-electron microscopy data collection and processing for this work was supported by the Cryo-Electron Microscopy Center of SUSTech.
Paper link: https://doi.org/10.15252/embj.2023114889
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo By