The human brain is an intricate network of interacting regions that work together to support perception, cognition, and behavior. Understanding how information flows between these regions—beyond just mapping physical connections or correlations in activity—remains a central challenge in neuroscience. While structural and functional connectivity offers valuable insights, they fall short of revealing the direction and causality of communication. Effective connectivity (EC) fills this gap by capturing causal interactions among brain regions, with implications for both basic science and clinical applications like neuromodulation.
In recent years, there has been growing interest in non-invasive computational methods for estimating EC. Among these, artificial neural networks (ANNs) have emerged as powerful tools for modeling brain activity. When integrated with perturbation analysis—a technique miming classical neuroscience approaches by systematically manipulating inputs and observing outcomes—ANNs offer a promising framework for inferring causal relationships in the brain at scale, overcoming some of the limitations of earlier model-based and data-driven methods.
The research team led by Assistant Professor Quanying Liu from the Department of Biomedical Engineering at the Southern University of Science and Technology (SUSTech) has proposed a data-driven approach for constructing an AI-based surrogate brain. By introducing a novel method, Neural Perturbational Inference (NPI), they inferred causal relationships within brain networks through virtual perturbation of the surrogate model.
Their study, titled “Mapping effective connectivity by virtually perturbing a surrogate brain”, has been published in the international journal Nature Methods.
The study introduces NPI, a data-driven framework for inferring EC that employs ANNs as digital twin brains to efficiently model brain activity and track interactions between regions. The process begins by training an ANN to predict brain activity at the next time point based on previous data, aiming to minimize prediction errors (Figure 1b). The ANN’s outputs are then recursively fed back into the model to generate synthetic neural signals (Figure 1e), which are compared with real resting-state fMRI (rs-fMRI) data. The high correlation between the model-derived FC (model FC) and empirical FC indicates that the ANN effectively captures functional dependencies between brain regions (Figure 1f). This supports the use of the trained ANN as a reliable digital twin brain for virtual perturbation.
To explore EC, the study perturbs each brain region of the ANN by applying a pulse signal to specific nodes (Figure 1c). This allows the researchers to infer the EC from the source region to the target region by comparing the activity changes under perturbed and baseline conditions. An increase in target activity indicates an excitatory EC, while a decrease implies an inhibitory EC (Figure 1h). By perturbing all nodes, the framework reconstructs EC across the entire brain (Figure 1d). The effectiveness of the NPI framework is validated using several generative models with known ground-truth EC (Figure 1i). When applied to rs-fMRI data, NPI reveals key features of human brain EC (Figure 1j).
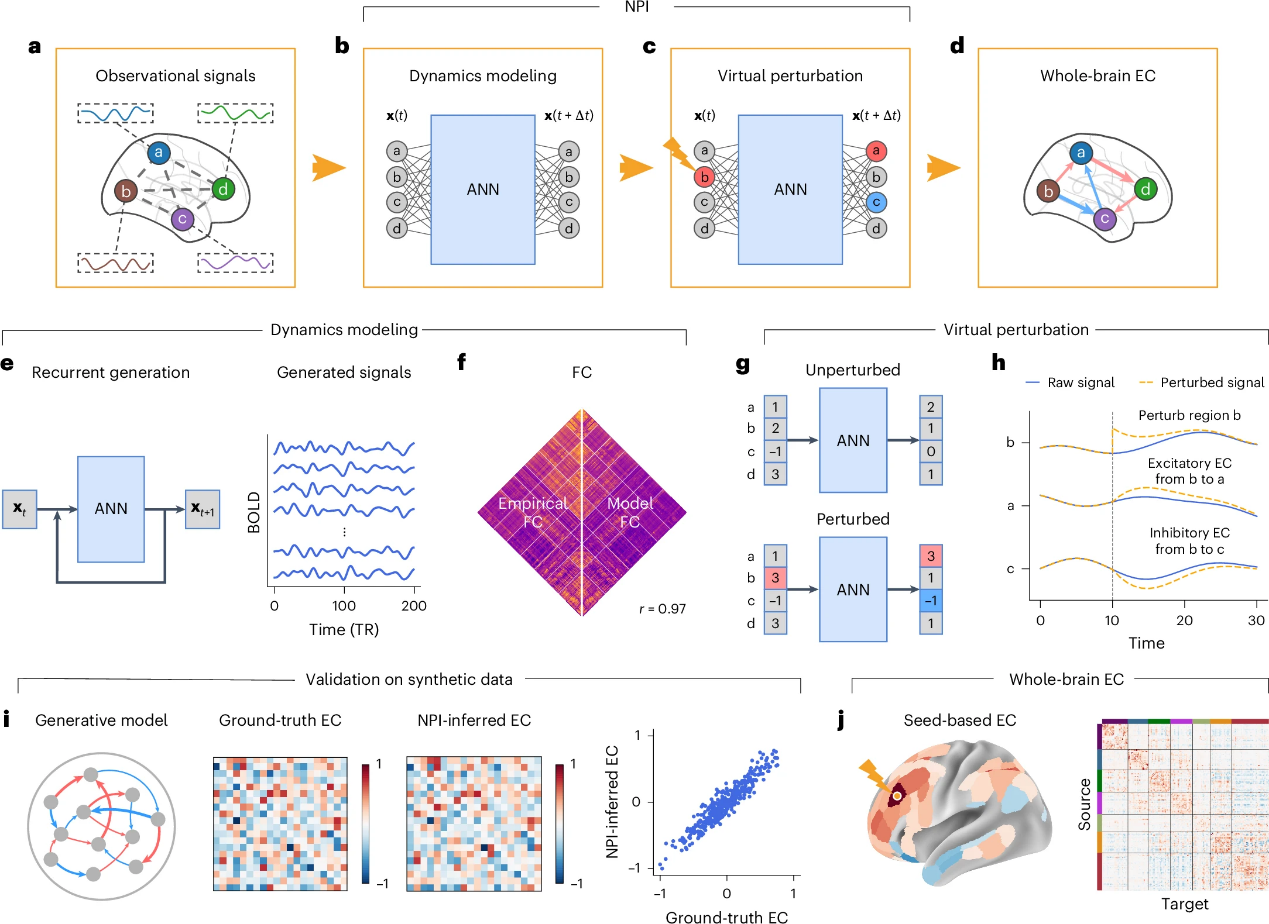
Figure 1. NPI infers EC by virtually perturbing AI-based surrogate brains
The research team evaluated the performance of NPI using three simulated datasets, including one generated by a recurrent neural network (RNN) model (Figures 2a-h) and two derived from generative models of fMRI (Figures 2i-p). The ground-truth EC is obtained by directly applying the “perturb-and-record” paradigm to these models. The EC inferred by NPI is then compared with the ground-truth EC to assess its accuracy.
Taking the RNN-based model as an example, an ANN is trained to replicate the dynamics of the RNN-generated data (Figure 2c). The model’s FC shows strong consistency with the empirical FC (Figure 2d), validating the model’s learning capability. NPI successfully infers EC from the trained ANN, showing a high correlation with the ground-truth EC (Figure 2f) and significantly outperforming traditional methods such as Granger causality (Figure 2g).
Further tests on simulated fMRI datasets demonstrate NPI’s robustness and accuracy across varying conditions, including different perturbation strengths, system noise, data length, and network size (Figures 2i-p). The results show that NPI maintains stable performance, particularly with sufficient data, and scales effectively to larger networks. The framework is also tested on complex fMRI dynamics, where it reliably identifies causal interactions between brain regions, demonstrating high accuracy and generalizability.
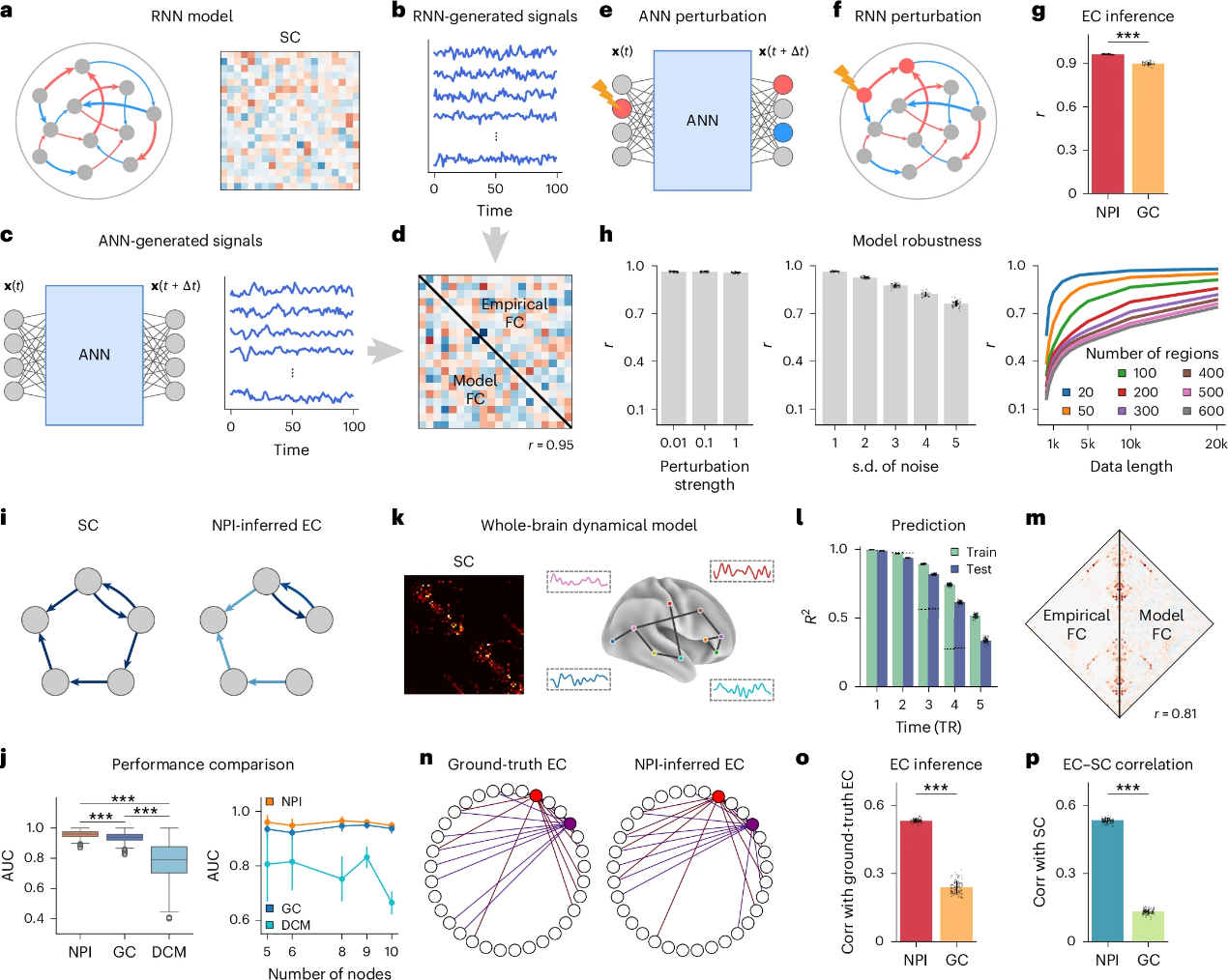
Figure 2. Validating the effectiveness of NPI on real data
NPI is applied to rs-fMRI data from 800 participants in the Human Connectome Project (HCP) dataset. For each participant, an individualized ANN is trained, using the Multi-Modal Parcellation (MMP) atlas to define brain regions. After comparing different input configurations, the study finds that using the previous three-time steps to predict the next step yields better performance than using a single time point. The model FC shows a strong correlation with the empirical FC (Figures 3c-d), supporting the use of this model. Based on this, a multi-layer perceptron (MLP) model with a three-step input is adopted for subsequent analysis. Perturbation analysis is conducted on each individualized ANN, and the inferred EC matrices are averaged across subjects to construct a population-level effective brain connectome (EBC), which captures both excitatory and inhibitory connections. (Figure 3a).
The analysis of the EBC reveals that the strengths of excitatory and inhibitory EC follow log-normal distributions (Figures 3f-g). The strongest excitatory connections are primarily found within functional networks, whereas the strongest inhibitory connections more often occur between networks and across hemispheres (Figures 3f-g). To assess the importance of each node within the network, the EBC matrix is binarized, and the node degree is computed. Nodes with high connectivity to other regions are broadly distributed across multiple functional networks, indicating a widespread distribution of high-centrality regions (Figure 3h).
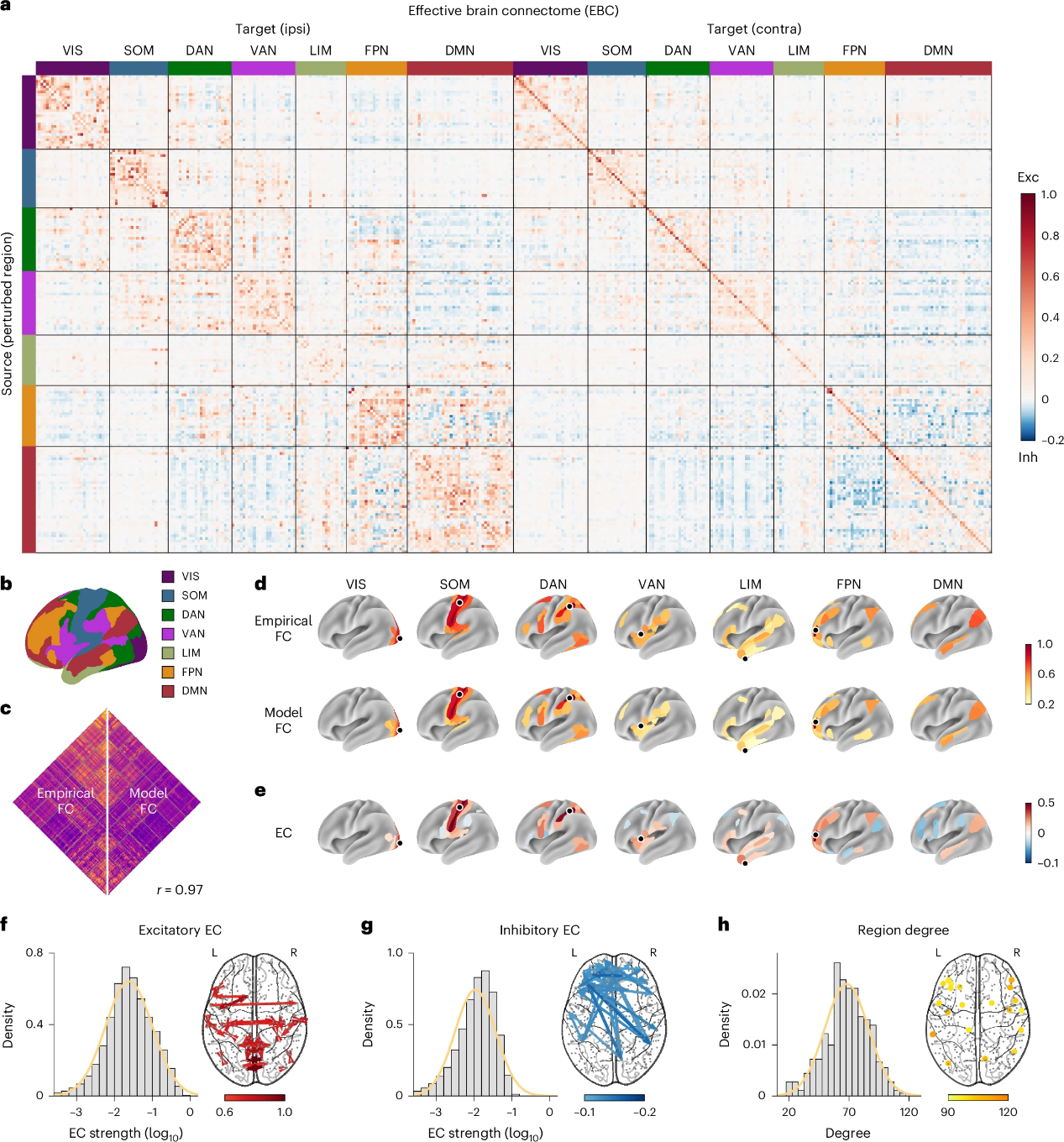
Figure 3. NPI produces the first effective connectome of the human brain
The researchers assess how the performance of NPI on fMRI data depends on the number of brain regions. The study applies NPI to multiple versions of the Schaefer atlas, with the number of parcels ranging from 100 to 1000 (Figures 4a-b). As the number of regions increases, predictive performance slightly declines, likely due to the increased data demands associated with finer parcellations (Figure 2h). Nonetheless, the model’s ability to recover FC remains stable across resolutions (Figure 4b), indicating that the NPI framework performs consistently across different spatial granularities.
In addition, they evaluate the robustness of NPI under various conditions, including different ANN initializations and fMRI scan segments across subjects and datasets (Figure 4c-e). The results show that NPI is robust to ANN initialization, reliably extracts individualized connectivity patterns, and produces reproducible results across datasets. The relationship between the NPI-inferred EBC and SC is also examined. SC derived from the HCP dataset is compared with the EBC obtained via NPI. The analysis reveals a significant correlation between SC and EBC (Figure 4f), highlighting the important role of anatomical structure in shaping functional communication in the brain.
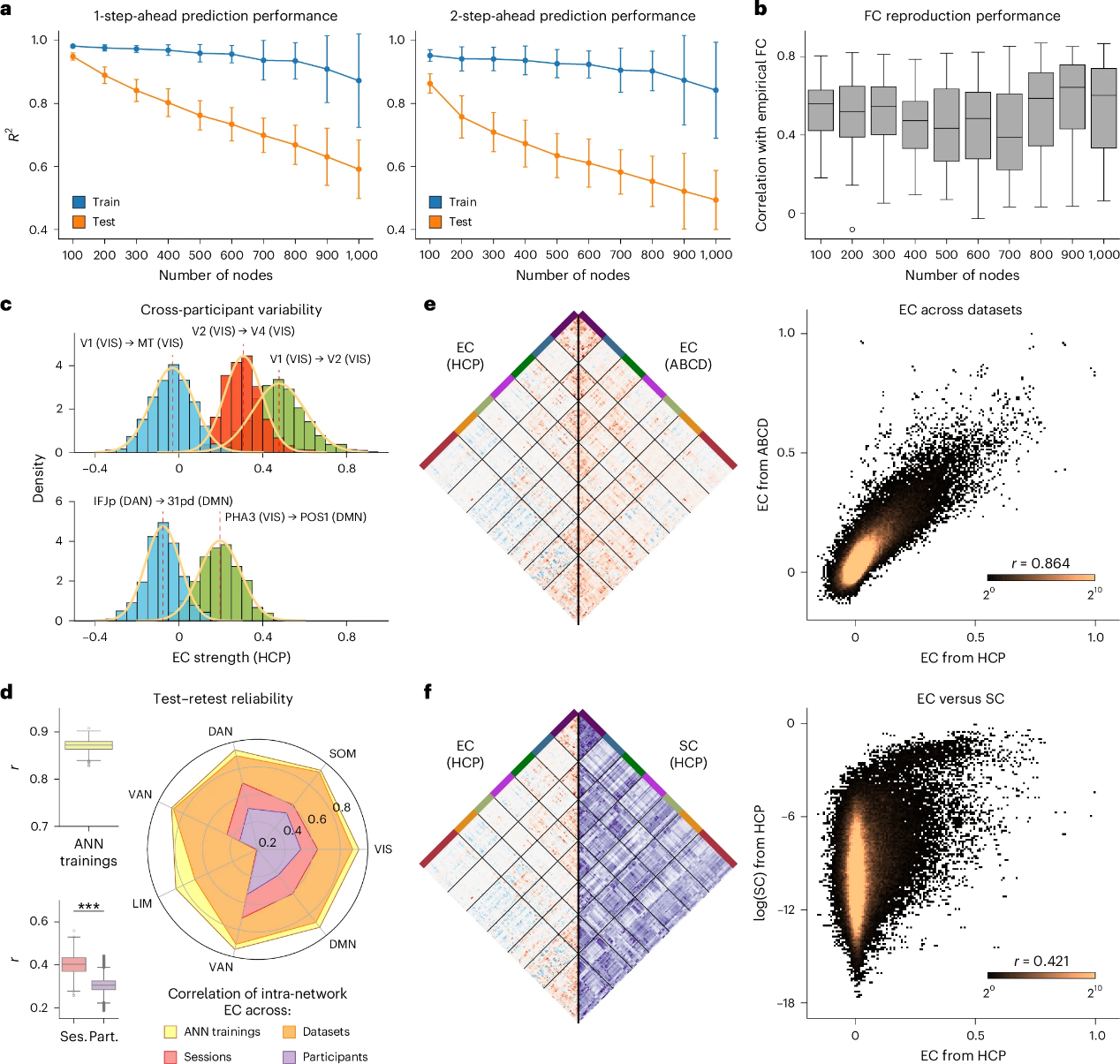
Figure 4. EC inferred by NPI is robust and aligned with SC
To evaluate the clinical potential of NPI, the team uses CCEP data provided by the F-TRACT project. This dataset includes a group-level CCEP connectivity matrix (Figure 5a) derived from stereo-electroencephalography (SEEG) recordings in patients with epilepsy (Figure 5b). The matrix reflects the propagation pathways of neural signals across cortical regions, making it an ideal reference for validating the EBC inferred by NPI. When comparing the population-averaged EBC derived from HCP data with the CCEP connectivity matrix, they find a significantly higher correlation between EBC and CCEP than between FC and CCEP. This suggests that the EBC inferred by NPI accurately captures the pathways of neural signal propagation following stimulation, revealing the brain’s underlying causal structure.
Further analysis explores the relationship between CCEP and both output and input EC inferred by NPI. It shows that NPI-derived EC closely matches CCEP’s spatial patterns and outperforms FC by a substantial margin (Figures 5f-g). Importantly, NPI offers key advantages over CCEP; it is non-invasive, uses only resting-state fMRI data, and provides broader generalizability and flexibility. These features make NPI a promising and accessible tool for research and clinical applications.
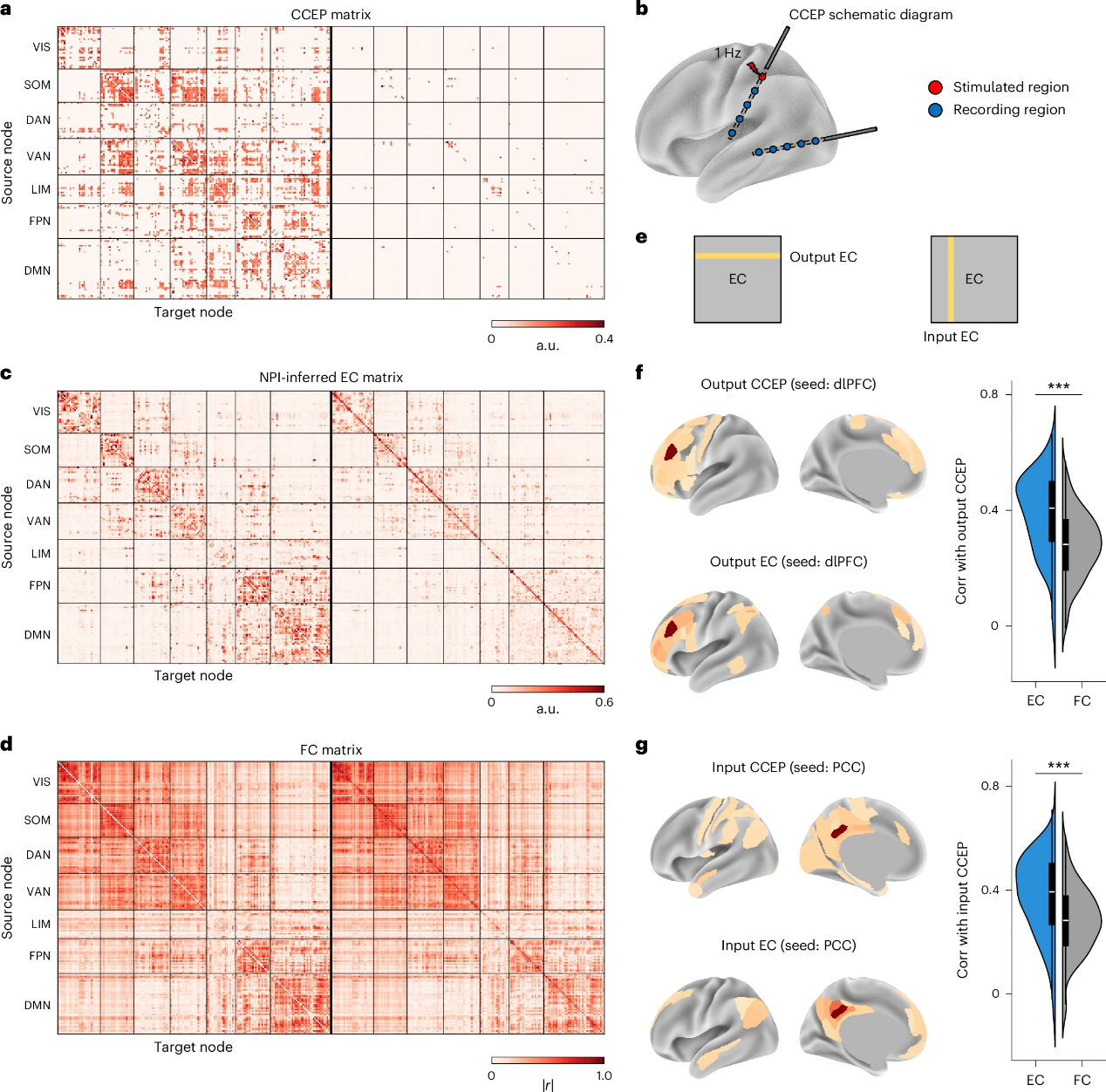
Figure 5. CCEP validates the EC inferred by the NPI framework
This study presents the NPI framework, which offers a novel, non-invasive approach for inferring whole-brain EC by simulating the classical “perturb-and-record” paradigm. Compared to FC, EC inferred by NPI captures causal interactions, revealing both the direction and regulatory nature of information flow between brain regions. In contrast to existing EC estimation methods, NPI demonstrates greater flexibility and generalizability, as it infers whole-brain causal maps using only rs-fMRI data without requiring assumptions about the underlying neural architecture.
The framework trains AI models to self-supervise the learning of brain network dynamics, allowing virtual perturbations to assess causal influences. Validated on simulated and rs-fMRI data, NPI highlights its potential for clinical translation. Nonetheless, it is sensitive to data quality and length, and its performance may degrade under conditions of short time series, high-dimensional parcellations, or low signal-to-noise ratio. Future improvements could involve applying NPI to task-based fMRI, EEG/MEG, and sparse recordings, as well as incorporating multimodal data and subject-specific fine-tuning strategies to enhance EC inference and expand its clinical utility.
Postgraduate student Zixiang Luo from the Department of Biomedical Engineering at SUSTech is the first author of the paper. Assistant Professor Quanying Liu is the corresponding author, and other authors from SUSTech include postgraduate student Kaining Peng, Dr. Zhichao Liang, and Research Assistant Shengyuan Cai.
Paper link: www.nature.com/articles/s41592-025-02654-x
Related link: https://github.com/ncclab-sustech/NPI
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yuwen ZENG
Photo ByDepartment of Biomedical Engineering