Aconitum species—medicinal treasures with complex and unresolved diterpenoid biosynthesis mysteries—are known for producing structurally diverse C19 and C20 diterpenoid alkaloids. These include pharmacologically active compounds such as the analgesic 3-O-acetylaconitine and antiarrhythmic guan-fu base A. Representative species such as Aconitum carmichaelii and Aconitum coreanum have long been used in traditional medicine, yet the biosynthesis of these structurally complex alkaloids remains enigmatic.
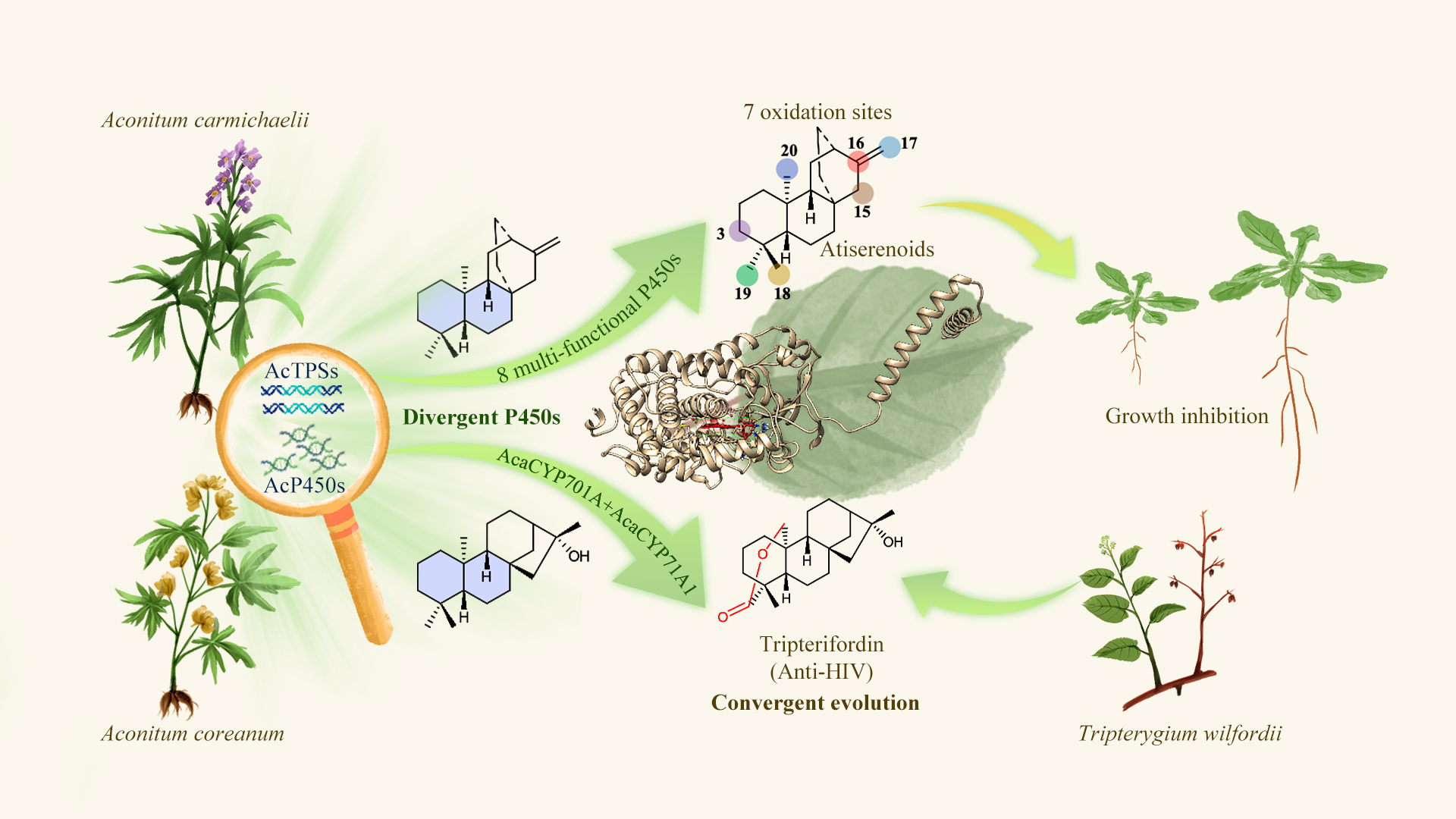
A research team led by Associate Professor Ancheng Huang from the School of Life Sciences at the Southern University of Science and Technology (SUSTech) has identified and characterized 14 functionally divergent cytochrome P450 oxidases from Aconitum genus transcriptomes. Among them, eight multifunctional P450s are capable of selectively oxidizing seven distinct sites on the labdane-type diterpenes (LRDs) skeleton, thereby driving structural diversification.
Through combinatorial biosynthesis, the team successfully achieved heterologous production of the anti-HIV compound tripterifordin and 14 novel diterpenoids. The study also reveals the convergent evolution of tripterifordin biosynthesis between Aconitum coreanum (Ranunculaceae) and Tripterygium wilfordii (Celastraceae), as well as the allelopathic potential of Aconitum diterpenoids as plant growth regulators. These findings lay the groundwork for elucidating downstream pathways of complex diterpenoid alkaloids (DAs).
Their paper, titled “Divergent multifunctional P450s-empowered biosynthesis of bioactive tripteritordin and cryptic atiserenoids in Aconitum implies convergent evolution”, has been published in Nature Communications.
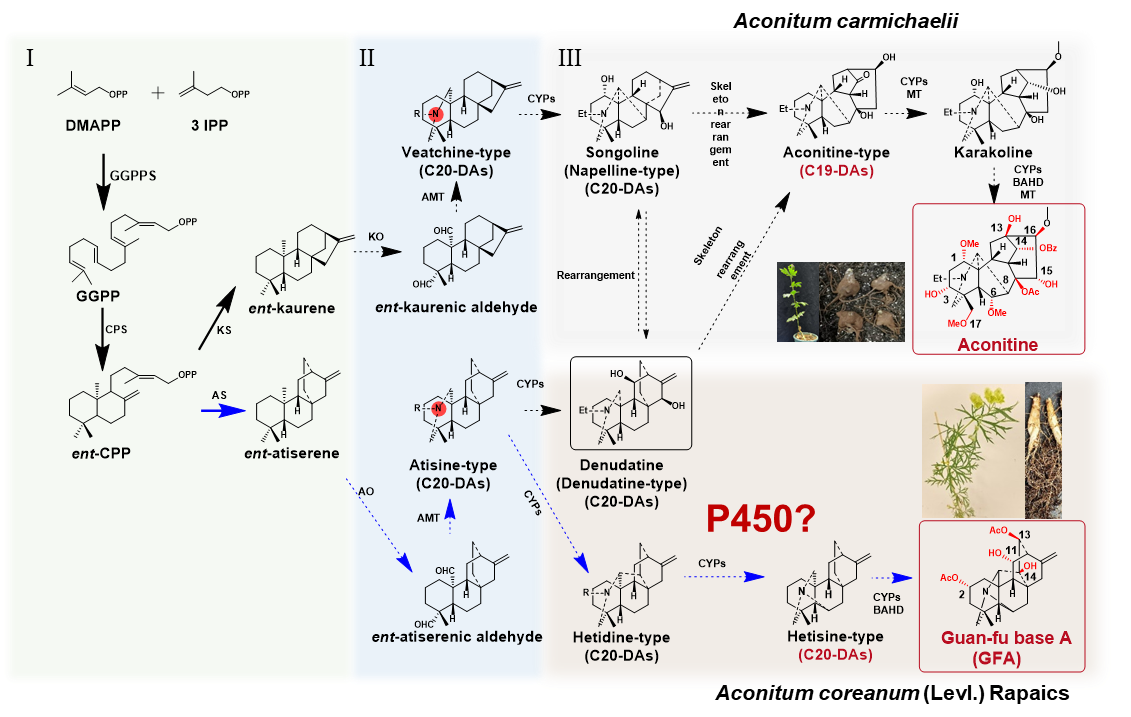
Figure 1. Overview of the modular biosynthetic pathways of the major bioactive constituents in A. carmichaelii and A. coreanum
The team identified nine diterpene synthases (DiTPS) from A. carmichaelii and A. coreanum, generating three skeletons: ent-atiserene, ent-kaurene, and 16α-hydroxy-ent-kaurene. Further screening revealed 14 functional P450s from the CYP71, CYP85, and CYP72 clans, which introduced oxidative modifications at seven positions (C3, C15, C16, C17, C18, C19 and C20) on the LRD skeletons. Among these, eight multifunctional P450s—primarily from CYP93A and CYP71A—catalyzed multiple-site oxidations.
Through combinatorial biosynthesis, the researchers yielded 19 oxidized products, including 14 novel atisane-type diterpenoids. These results highlight P450 plasticity in structural diversification (Figure 2). Using AlphaFold2-predicted structures and mutagenesis, they pinpointed key residues governing catalytic versatility.
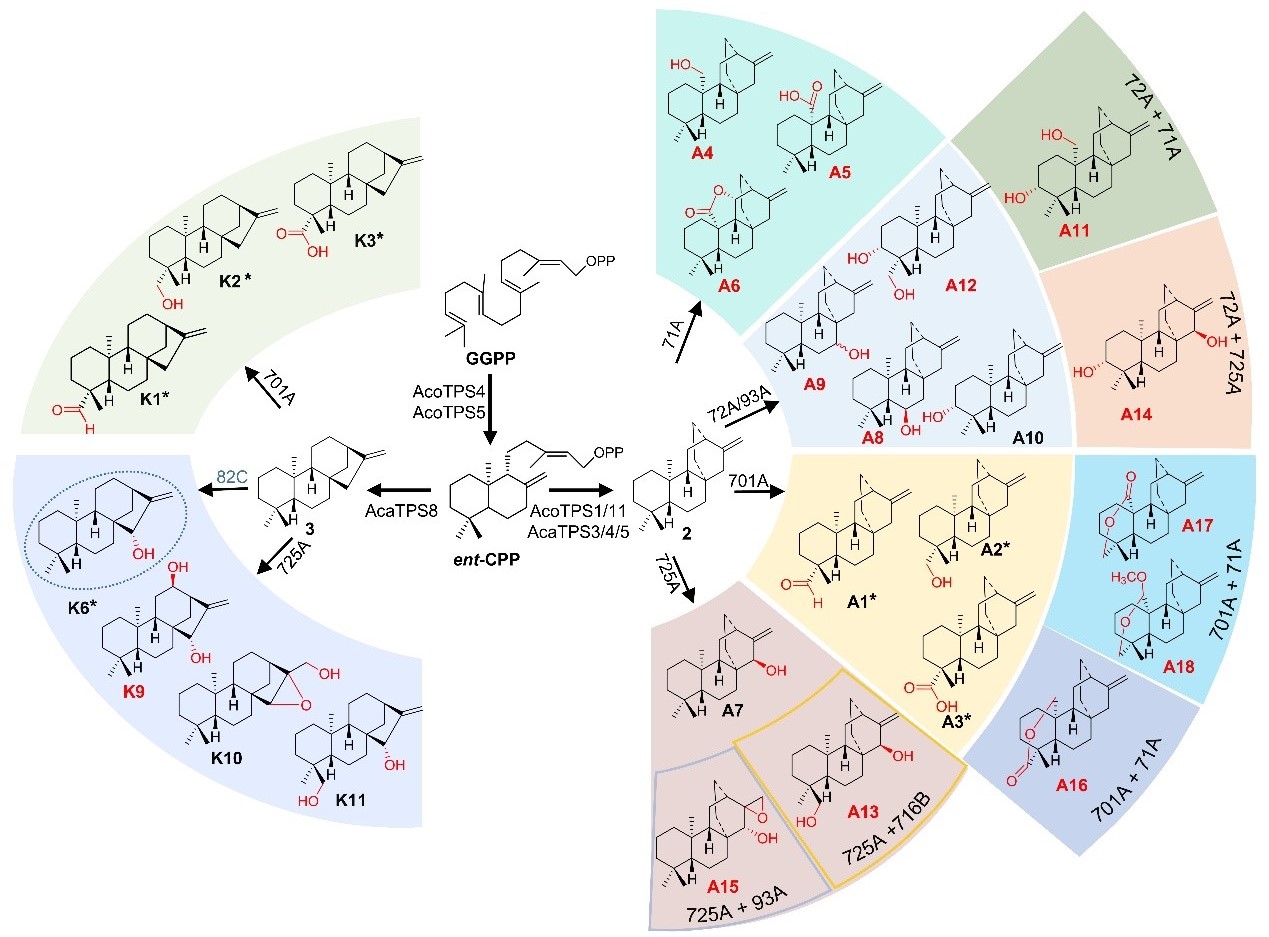
Figure 2. Divergent biosynthesis of labdane-related diterpenes driven by P450 enzymes in Aconitum plants
By combining AcoTPS3 (which produces 16α-hydroxy-ent-kaurene, a tripterifordin precursor) with two P450s—AcaCYP71A1, AcoCYP701A2—the team heterologously synthesized tripterifordin (T7) and guan-fu diterpenoid A (T1) in tobacco (Figure 3). Intriguingly, tripterifordin was detected in A. coreanum tubers at levels of 1.2 mg/g dry weight, demonstrating convergent evolution between A. coreanum and T. wilfordii, despite the two species diverging approximately 13 million years ago.
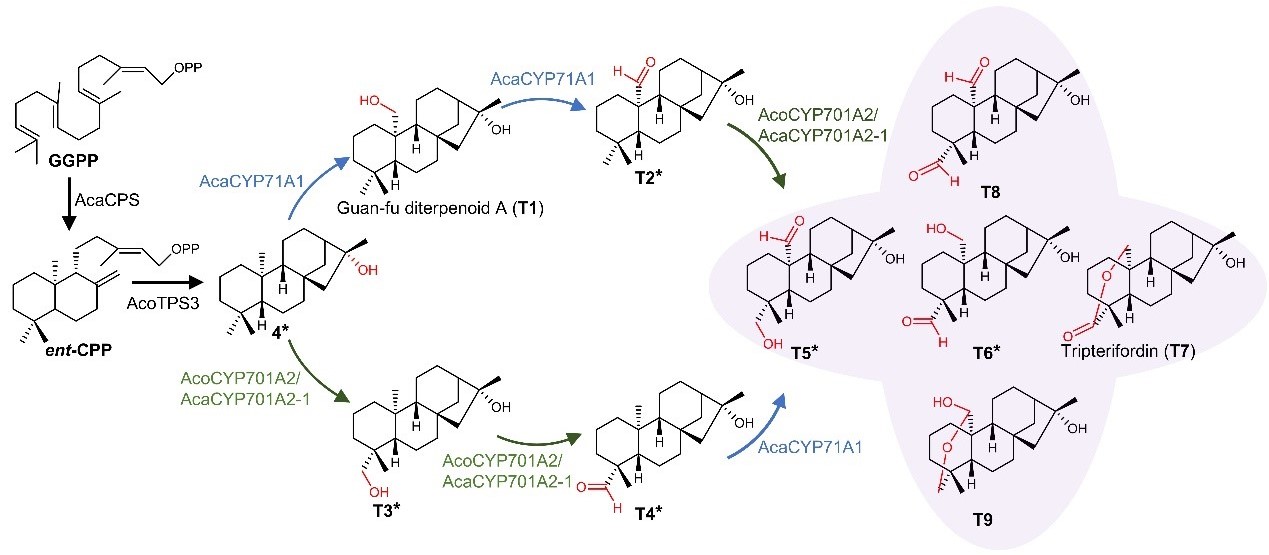
Figure 3. Heterologous biosynthesis of tripterifordin T7 and other diterpenoids based on the TPS and P450 enzymes of the Aconitum genus
Several newly identified atisane-type diterpenoids—including ent-atiselenoids A10, A11, A13, and A15—as well as tripterifordin, suppressed Arabidopsis growth, akin to paclobutrazol (PAC), suggesting ecological roles as allelochemicals (Figure 4). These findings underscore Aconitum diterpenoids’ potential as plant growth regulators.
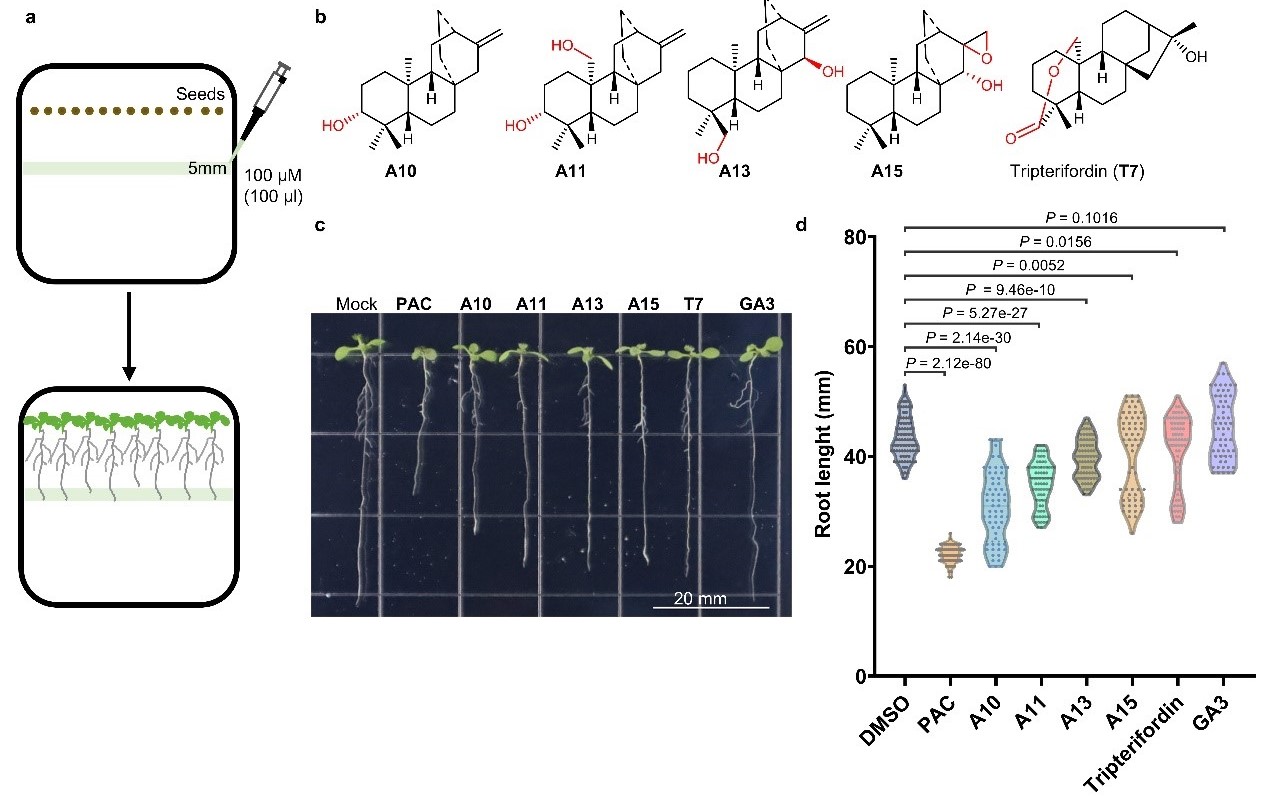
Figure 4. Diterpenoids of the Aconitum genus exhibit growth-inhibitory activities against plants
Ph.D. graduate Fei Luo from Associate Professor Ancheng Huang’s group and Research Assistant Professor Qian Zhou are the co-first authors of the paper. Associate Professor Huang is the corresponding author, with SUSTech serving as the primary affiliated institution. Other contributors to this study include former group members Dr. Feilong Chen and Dr. Xinyu Liu, Associate Professor Guoyuan Zhu from the University of Macau, and Dr. Canyu Qiu from BGI-Research.
Paper link: https://www.nature.com/articles/s41467-025-61188-0
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yuwen ZENG
Photo BySchool of Life Sciences