Robust methods in organic synthesis are fundamental to the creation of new molecules, enabling advances across chemistry and related disciplines. A central goal in synthetic chemistry is the development of reactions that combine high yields with broad substrate scope, thereby maximizing reaction generality. Achieving this level of generality in catalytic asymmetric reactions is particularly challenging, as optimizing for both selectivity and yield often involves addressing complex issues of enantioselectivity. The challenge is especially pronounced for very reactive species such as radicals, where the control of stereochemistry remains formidable.
Traditional approaches to catalytic asymmetric radical bond-forming processes—including coupling, addition, and substitution—typically rely on stereodiscrimination between a prochiral radical or its reaction partner and the catalyst. However, the activation barriers for these processes are generally low, which makes it challenging to achieve sufficient energy differences between competing diastereomeric transition states. As a result, catalytic reactions involving highly unstable and reactive radicals often exhibit poor or marginal enantioselectivities. Notably, to date, there have been no credible examples of asymmetric transformations involving even less stable radicals such as tert-butoxy or phenyl radicals. These limitations highlight an urgent need for catalytic asymmetric radical platforms that couple broad generality with high levels of enantiocontrol.

A collaborative team from the Department of Chemistry at the Southern University of Science and Technology (SUSTech)—Chair Professor Xin-Yuan Liu, Associate Professor Qiang-Shuai Gu, and Associate Professor Zhe Dong—together with Professor Xin Hong at Zhejiang University, has achieved an important advance in the asymmetric transformation of highly reactive radicals.
Their work, titled “Copper-catalysed asymmetric cross-coupling reactions tolerant of highly reactive radicals,” has been published in the international journal Nature Chemistry.
Xinyuan Liu’s team has long been dedicated to the research of radical asymmetric catalytic reactions. In recent years, they have developed a copper-catalyzed asymmetric radical cross-coupling strategy, achieving efficient asymmetric transformation of a series of radicals (Nature 2023, 618, 294; Science 2025, 388, 283), including substantive advances with highly reactive alkenyl and cyclopropyl radicals. Building on this foundation, they devised a sequential stereodiscrimination and chirality transfer strategy to address the stereorecognition challenges inherent to highly reactive radicals.
In this approach, a prochiral or racemic substrate first forms a catalyst-bound complex, thereby establishing a robust stereocentre in the substrate under conditions in which the relevant species are relatively stable. This process facilitates stereocontrol at an early stage, before the engagement of highly reactive radicals. Subsequently, the catalyst-bound intermediate undergoes a radical reaction with high stereochemical fidelity, which may proceed with either retention or inversion of the established stereocentre, resulting in overall excellent chirality transfer.
As the steps leading to the catalyst complex typically involve higher activation barriers than the subsequent rapid reactions with highly reactive radicals, achieving a sufficiently high stereoselectivity for a specific diastereomer during complex formation becomes more feasible. This selectivity ensures that only the desired stereoisomer participates in the subsequent radical reaction, while undesired pathways are effectively suppressed. Thus, this strategy leverages the stereodiscrimination of relatively stable intermediates, rather than highly reactive radical species, to achieve efficient and selective stereocontrol in otherwise challenging radical transformations.
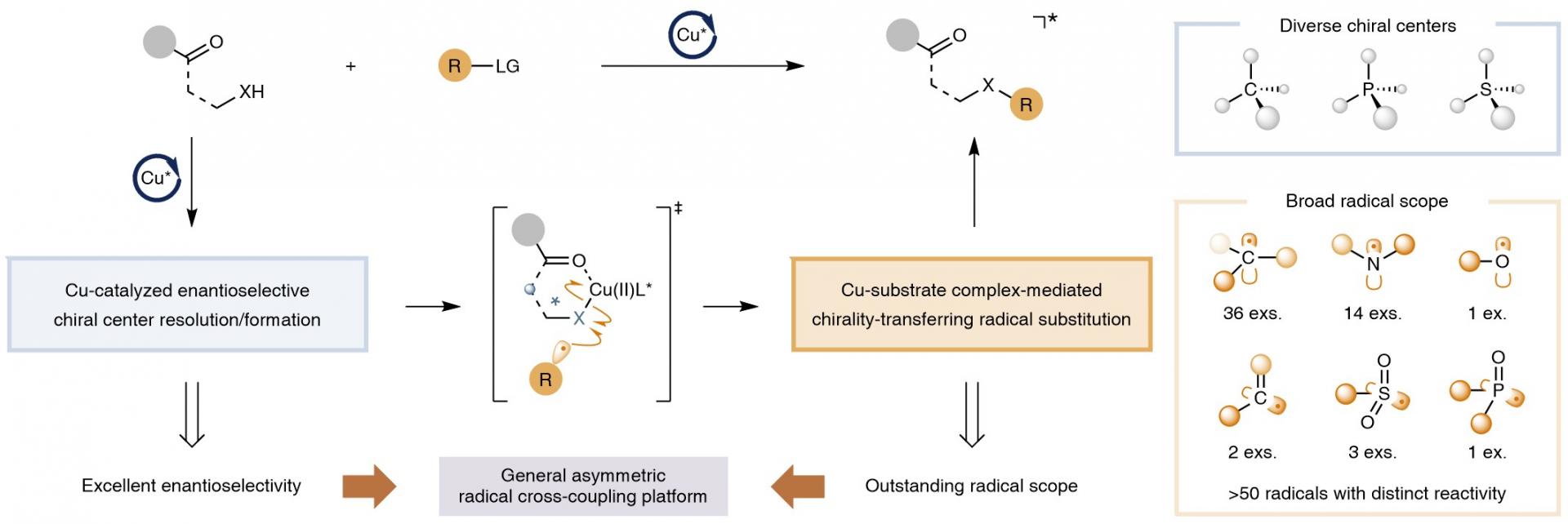
Figure 1. Copper-catalysed asymmetric cross-coupling reactions tolerant of highly reactive radicals
In this study, the researchers report the development of a highly versatile and general catalytic asymmetric radical cross-coupling of γ-aminocarbonyl alcohols, β-aminocarbonyl H-phosphinates, and N-acyl sulfenamides with a diverse array of electrophiles (Figure 1). This method accommodates over 50 different C-, N-, O-, S-, and P-centered radicals—including highly reactive methyl, tert-butoxyl and phenyl radicals—enabling access to a wide variety of enantioenriched C-, P- and S-chiral compounds.
Importantly, this synthetic strategy enables the rare catalytic stereoselective synthesis of a prominent anti-cancer drug, fulvestrant, which has hitherto been commercialized in its diastereomeric mixture form due to the aforementioned synthetic challenges. The team anticipates that this asymmetric radical coupling approach will be broadly applicable to the synthesis of both heteroatomic and carbon stereocentres, thereby advancing the field of asymmetric radical chemistry.
Research Assistant Professor Li-Wen Fan, doctoral graduate Li-Lei Wang, and former postdoctoral fellows Jun-Bin Tang, Zeng Gao, and Ji-Ren Liu from the Department of Chemistry at SUSTech are co-first authors of the paper. Chair Professor Xin-Yuan Liu, Associate Professor Qiang-Shuai Gu, Associate Professor Ze Dong, and Professor Xin Hong are co-corresponding authors, and SUSTech is the primary and corresponding affiliated institution.
Paper link: https://doi.org/10.1038/s41557-025-01970-1
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yifei REN
Photo ByYan QIU