Atropurpuran is from a class of natural chemical compounds that is thought to relieve pain, particularly in folk medicine in the northern hemisphere. A new study from Southern University of Science and Technology (SUSTech) has found a more efficient way to synthesize this unique compound, thereby reducing the reliance on previous methods that were more time-consuming.
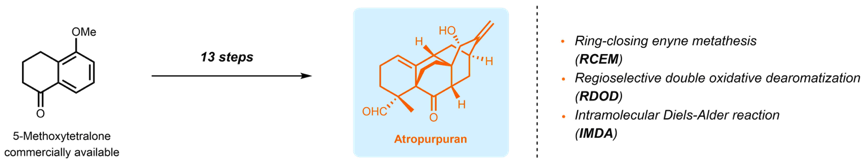
Associate Professor of Chemistry Xu Jing has led his research team to make important progress in natural product synthesis. Their research was examining a new efficient strategy for the total synthesis of atropurpuran, which had previously been synthesized in 25 steps. The team believed that they could achieve it in much fewer steps, and their 13 step process was published in the Journal of the American Chemical Society under the title of “13-Step Total Synthesis of Atropurpuran,” on 13 February 2019. The co-first authors were research assistants Xie Shengling and Chen Gui and postdoctoral researcher Yan Hao. They were assisted by research assistant Hou Jieping, postdoctoral researcher He Yongping and undergraduate Zhao Tongyun.
Atropurpuran is a type of diterpenes, which are organic chemical compounds with a unique structure and intriguing bioactivity. They also feature complex structures, which make them extremely difficult to synthesize. Meanwhile, they fall within a family of natural products that share a common organic carbon structure. Their structure has attracted a lot of attention from researchers all over the world as their biological properties remain locked at this point.
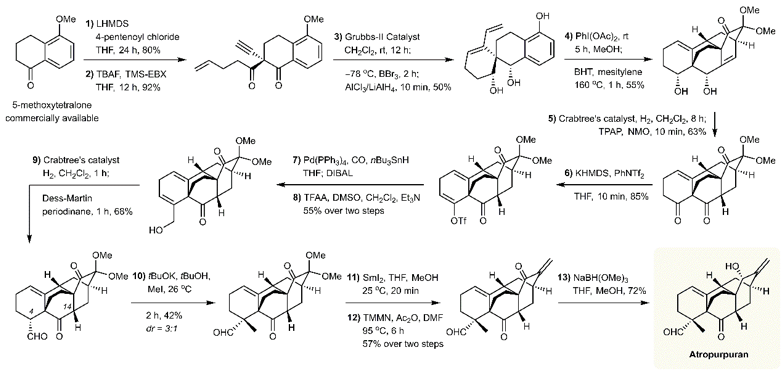
Xu Jing’s team went through an extensive series of experiments over almost four years that required the application of various synthetic methods, organic reagents and catalysts to ensure the synthetic strategy would proceed as hoped.
At the end of their project, the team found that they had generated synthetic atropurpuran that was identical in all aspects to both natural and synthetic atropurpuran. Their synthetic strategy is highly concise and efficient,, so the team hopes that this strategy could inspire researchers to generate more natural and unnatural analogues of atropurpuran in the future.
Associate Professor Xu Jing said “The fundamental goal of this area of cutting-edge science of drug synthesis is to start from a simple, economical, and readily available raw material. The goal is get to the final product through the least number of steps, the highest yield, and the best route. This is what high-efficiency chemical synthesis ultimately reflects.”
When this is achieved, it means a reduction in costs, whether that is time or production and that means cheaper medicine for society. It also means less environmental pollution from the manufacture of medicine and less medical waste. Thus, high-efficiency synthesis of complex drug molecules and natural products with medicinal prospects is an area of heavy research in organic chemistry. That is why the team has been pursuing this goal.
The research team plans to continue conducting high-efficiency research into the synthesis of a variety of complex drug molecules and biologically active natural product molecules. They will be looking for the medicinal prospects of those natural product molecules, with the aim of contributing to the cutting edge of medical science in China.
This research has been financially supported by NSFC, SZSTI, SZDRC Discipline Construction Program and Shenzhen Nobel Prize Scientists Laboratory Project. The authors greatly thank the support of SUSTech and, especially President Shiyi Chen.
Original paper: https://pubs.acs.org.ccindex.cn/doi/10.1021/jacs.9b00391
Proofread ByXia Yingying
Photo ByDepartment of Chemistry