The genus Daphniphyllum comprises evergreen trees and shrubs native to the Asia-Pacific region, which have long been used in traditional Chinese medicine. Various Daphniphyllum alkaloids have exhibited diverse bioactivities, such as antitubulin polymerization, anticarcinogenic, anti-HIV, vasorelaxant, and cytotoxic activities. More than 330 Daphniphyllum alkaloids have been isolated from the genus Daphniphyllum to date, comprising a structurally fascinating and diverse natural product family. From a chemical structure perspective, these alkaloids can be categorized into over a dozen subfamilies on the basis of their distinct structural backbones. Highly challenging and congested polycyclic ring systems, along with promising bioactivities, make these alkaloids intriguing synthetic targets.
Recently, Associate Professor Xu Jing from the Department of Chemistry at Southern University of Science and Technology (SUSTech) led his research team to successfully complete the enantioselective total synthesis of Dapholdhamine B and its lactone derivatives for the first time. This is also the first synthesized aza-adamantane natural product. This achievement was recently published in the top international journal of chemical science J. Am. Chem. Soc. (the American Chemical Society). SUSTech is the only communication unit, and Associate Professor Xu Jing is the sole correspondent.
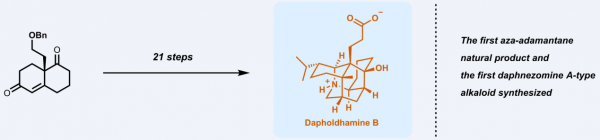
Dapholdhamine B is a Daphnezomine A alkaloid isolated by Professor Hao Xiaojiang, Kunming Institute of Botany, Chinese Academy of Sciences, in 2009. Dapholdhamine B has a very rare and unique structure of aza-adamantane, and contains eight chiral centers and three successive full-substituted quarterly carbon centers. Its chemical synthesis is very challenging, and there is no report on the synthesis of the alkaloids with related skeletons.
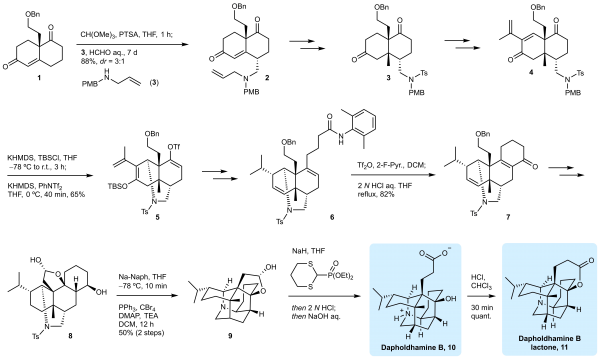
The research team led by Associate Professor Xu Jing was able to accomplish the total synthesis Dapholdhamine B in just 21 steps. The highly complex substrate skeleton structure meant that a wide variety of approaches needed to be taken in order for the successful synthesis to occur. Because the natural product is an amino acid structure, its NMR spectra are very sensitive to the pH value, which cannot be compared with the literature spectra. Therefore, Xu Jing’s team transformed Dapholdhamine B into its lactone derivative 11. The first synthesis of Dapholdhamine B has been successfully confirmed by extensive NMR analysis of 11 and unambiguous structural characterization of the last intermediate 9 (X-ray single crystal diffraction). This is also the first synthesized aza-adamantane natural product.
Adamantane has previously been found in petroleum, and its discovery led to a unique branch of chemistry. Potential applications of aza-adamantane could include drugs, polymeric materials and lubricants.
Senior research scholars Dr. Guo Liandong, Hou Jieping and Tu Wentong conducted the experiments for this research, along with masters students Zhang Yan and Zhang Yue and undergraduate student Chen Louxi. Xu Jing’s research group is very grateful to SUSTech, the Shenzhen Grubbs Institute, the National Natural Science Foundation of China, Shenzhen Nobel Prize Scientists Laboratory Project, the Shenzhen Development and Reform Commission and the Science, Technology and Innovation Commission of Shenzhen Municipality for their strong support in research platform and funding.
Links to papers: https://pubs.acs.org/doi/abs/10.1021/jacs.9b05641
Proofread By
Photo ByDepartment of Chemistry