The synthesis of natural products that feature good biologically active applications have been the source of significant research, but their often congested organic structures make their chemical synthesis extremely challenging. A group of researchers in Shenzhen has been working on this challenge for many years, and has found a number of unique solutions.
2019 has been an excellent year of research for Associate Professor Xu Jing and his research group at the Department of Chemistry at Southern University of Science and Technology. He and his group has been published in the Journal of the American Chemical Society (JACS) (IF = 14.7) for their research into atropurpuran & aza-adamantane natural products, and Angewandte Chemie International Edition (Agnew Chem Int Ed) (IF = 12.3) for their research into natural alkaloid synthesis. In recent days, the team has been published in JACS for the progress they made in the synthesis of structurally complicated natural products. The paper was titled “Enantioselective Total Synthesis of (−)-Caldaphnidine O via a Radical Cyclization Cascade,” in which they reported the first total synthesis of the complex caged alkaloid Caldaphnidine O.

The Daphniphyllum alkaloids were isolated from the plants of the genus Daphniphyllum, which are widely distributed in China, New Guinea and Japan. They have a highly complex and variable ring skeleton structure. Since the first isolation of daphnimacrine, more than 330 species of alkaloids have currently been found.
Traditional Chinese medicine believes that the plants of this genus can clear away heat, detoxify the body, promote blood circulation, relieve phlegm and pain, treat sore swollen poison and other ailments. Modern scientific research has also found that some of the Daphniphyllum alkaloids also have important properties in treating life-threatening diseases such as cancer, HIV and neurological disorders. As a result, the Daphniphyllum alkaloids have attracted significant attention from synthetic chemists.
The research team of Associate Professor Xu Jing used rapid skeleton construction and high selective chemical reactions as the core strategy, and efficiently completed the total synthesis of several quite challenging complex Daphniphyllum alkaloids. In their study of the total synthesis of Dipholdhamine B, the researchers accidentally discovered a 1,5-hydrogen transfer reaction, which inspired the design for high-efficiency free radical tandem cyclization for the synthesis of Caldaphnidine O. From there, they were able to complete the first total synthesis of Caldaphnidine O in just 15 steps. As a result, future researchers have a set of innovative ideas and strategies for the efficient synthesis of other complex natural products.
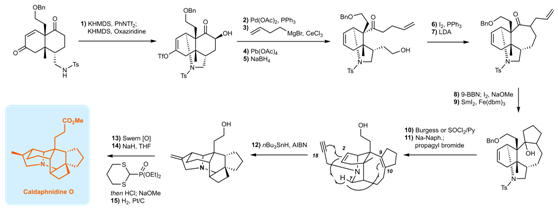
Figure 2: Schematic diagram of the synthetic route
This synthesis better guarantees the efficiency of the synthetic route by rapid skeleton construction and highly selective chemical reactions, as discussed in a review article in Nature Reviews Chemistry earlier this year by Associate Professor Xu Jing.

SUSTech is the only communication unit and Associate Professor Xu Jing is the sole correspondent. The research work was carried out by Dr. Guo Liandong, Ph.D. student Hu Jingping, masters’ student Zhang Wei, research assistant Tu Wentong, master student Zhang Yue and SUSTech undergraduate Pu Fan.
The research group received financial support from the NSFC, SZDRC Discipline Construction Program, Shenzhen Nobel Prize Scientists Laboratory Project and SZSTI. The authors also thank Dr. Xiaoyong Chang (SUSTech) for single crystal X-ray diffraction analysis.
Proofread ByXia Yingying
Photo ByDepartment of Chemistry