Recent internationally collaborative research between Southern University of Science and Technology (SUSTech) and Yale University could lead to the conversion of carbon emissions to renewable fuel and other value-added chemicals, according to a paper published in Nature today.
The teams of Professor Yongye Liang from the Department of Materials Science and Engineering at SUSTech and Professor Hailiang Wang from the Department of Chemistry at Yale University have worked together in significantly improving electrocatalytic reduction of carbon dioxide. Their paper was published in Nature under the title of “Domino electroreduction of CO2 to methanol on a molecular catalyst.”
The use of electrical energy generated by renewable energy to reduce carbon dioxide (CO2) has been considered as a sustainable method to produce chemicals and fuels. The key to this conversion is a CO2 reduction electrocatalyst, but current materials lack energy conversion efficiency and atomic economy.
Molecular catalysts have been the focus of many studies due to their distinct structure of active sites and easy regulation of molecular properties. But they often perform worse than the noble metal catalysts such as gold or silver, and can generally only achieve a two-electron reduction (for example from CO2 to carbon monoxide (CO)).
Previous research by the teams of Professor Yongye Liang and Professor Hailiang Wang (published in Nature Communications, 2017, 8, 14675) developed a method to make the cobalt phthalocyanine (CoPc)/carbon nanotube (CNT) hybrid by anchoring CoPc molecules on the side walls of CNTs. It successfully overcame the aggregating and low-conductivity problems of CoPc molecules, which greatly improved the electrocatalytic performance for CO2 reduction to CO.
The work published in Nature today has found, in a world first, that the CoPc/CNT hybrid catalyst can efficiently achieve a six-electron reduction, from carbon dioxide to methanol (CH3OH) at larger overpotentials than the previous experiment (Fig. 1). They were able to convert carbon dioxide to methanol at 40% of Faradic efficiency, reflecting the first example of molecular electrocatalysts for efficient carbon dioxide reduction to methanol.
The researchers found that the superior performance of CoPc/CNT benefits from the moderate binding energy of carbon monoxide on CoPc. The dispersion of CoPc molecules on CNTs significantly improved the catalytic performance through increased selectivity and partial current density compared to the physical mixtures of CoPc and carbon materials.
The generation of methanol in the reaction resulted in a reduced carbon monoxide yield, inspiring the research teams to consider that carbon monoxide is the intermediate of the CO2 to CH3OH conversion. They found that CoPc/CNT could also effectively reduce CO to CH3OH at 28% of Faradic efficiency, supporting that the electrocatalytic reaction of CO2 to CH3OH is, in fact, a Domino process: CO2 is first reduced to CO, which is then reduced to CH3OH.
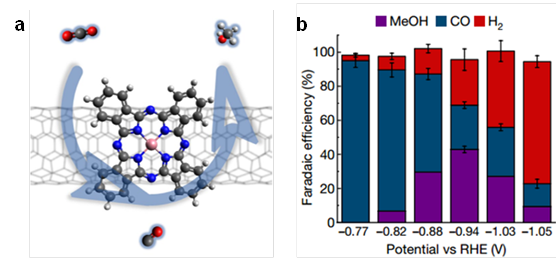
Figure 1: (a) CoPc/CNT catalyzed domino reduction of CO2 to methanol; (b) Reaction selectivity at different reaction potentials.
The team noticed an instability problem for CoPc/CNT when catalyzing CO2 to CH3OH, as a long reaction time would detrimentally reduce the CoPc molecules at large overpotentials and make the reactivity of CH3OH conversion decayed substantially (Fig. 2). To solve this problem, the researchers introduced four electron-donating amino groups (NH2) on the phthalocyanine ring to make CoPc-NH2/CNT hybrid (Fig. 2a), affording a lower reduction potential and increased structural stability. After 12 hours of reaction, the Faradic efficiency of CH3OH production on CoPc-NH2/CNT still maintained at 28%, similar to the original 32% (Fig. 2c).
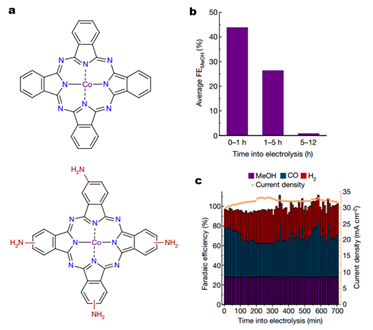
Figure 2: (a) Molecular structures of CoPc and CoPc-NH2; (b) methanol selectivity change of CoPc/CNT at -0.94V for 12 hours; (c) Product analysis and reduction current of CoPc-NH2/CNT at -1.00V for 12 hours.
Yale University graduate student Yueshen Wu is the first author of the paper. SUSTech graduate student Zhan Jiang is the second author, and the third author is Yale University postdoctoral researcher Dr. Xu Lu. Professor Yongye Liang and Professor Hailiang Wang are the correspondent authors of the paper.
This work was supported by the US National Science Foundation, a Croucher Fellowship for Postdoctoral Research, Shenzhen Fundamental Research Funding and the Guangdong Provincial Key Laboratory. The paper has obtained the help of Professor Meng Gu and postdoctoral researcher Dr. Qi Wang for assistance with STEM characterization.
Link to the paper: https://www.nature.com/articles/s41586-019-1760-8
Proofread ByXia Yingying
Photo ByDepartment of Materials Science and Engineering