The first total synthesis of a bioactive natural product could lead to improved anti-inflammatory drugs in the future, thanks to research led by Southern University of Science and Technology (SUSTech).
Professor Chuangchuang Li (Chemistry) led his research group to publish a paper in the high-impact academic journal, Journal of the American Chemical Society (JACS) (IF = 14.612). Their paper was titled “Asymmetric Total Synthesis of Bufospirostenin A.” The team used a novel reaction as the vital step to complete the efficient total synthesis of Bufospirostenin A in 20 steps. It is also the first time the alkoxyallene-yne Pauson-Khand reaction was applied to the total synthesis of natural products.
The small molecule metabolites of organisms are called natural products. Most of these compounds have diverse structures and have important essential physiological activities. They are a vast treasure trove of new drugs. Steroids are widely found in animals, plants, and insects, playing a vital role in regulating the growth and development of organisms.
The first discovered steroid natural product, cholesterol, is essential for maintaining the normal physiological functions of the human body. Due to their biological importance and structural diversity, steroids always play an important role in synthetic organic chemistry and drug discovery. Therefore study on the synthesis of complex active steroid natural products has been a hot issue and frontier in synthetic chemistry for a long time.
Bufospirostenin A is a sterol natural product isolated from the venom of the traditional Chinese medicinal material—Bufo bufo gargarizans. With only 1.9 mg (0.004 mmol) available, Bufospirostenin A has been reported to possess a cardioactive effect and promote blood circulation by causing a 43% inhibition of Na/K ATPase (NKA) at 25 μM.
The research group used readily available chiral derivatives as starting materials. The first five steps of conversion are the precursor of the Pauson—Khand reaction. The Pauson-Khand reaction was conducted with carbon monoxide and occurred under rhodium (Rh) catalysis.

It resulted in the group obtaining the 5/7/6/5 tetracyclic core skeleton of Bufospirostenin A with an excellent yield. However, several factors make the Pauson—Khand reaction extremely challenging. These include the instability of the electron-rich allene, interference of the exposed hydroxyl group, and the unfavorable entropy change in the ring closure.
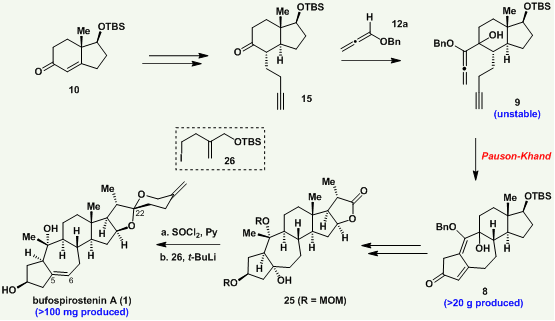
A series of redox, carbon-increasing reactions, along with the use of protecting groups, promoted the construction of the skeleton and oxidation state. The final 1,2 addition of carbonyl group and acid-catalyzed spirocyclization completed the total synthesis of Bufospirostenin A.
Their continued research into their synthetic sample of Bufospirostenin A showed that it held high-quality anti-inflammatory properties. It, therefore, held enormous potential for the pharmaceutical industry. Their synthetic methodology could also be applied to the asymmetric synthesis of other natural products that contain the [5/7/6/5] tetracyclic framework.
Jinan University and SUSTech joint doctoral candidate Minjing Cheng and SUSTech doctoral student Liping Zhong are the co-first authors. Professor Chuangchuang Li is the corresponding author of the paper, with Jinan University Professor Wencai Ye and Lei Wang as co-corresponding authors. Additional contributions came from SUSTech undergraduate student Chenchen Gu, visiting student Xujiang Zhu, and Shenzhen Grubbs Institute Research Assistant Professor Bo Chen.
This work was supported by the National Natural Science Foundation of China, the Shenzhen Science and Technology Innovation Committee, the Shenzhen Nobel Prize Scientists Laboratory Project, and supported by Shenzhen Peacock Plan.
Proofread ByYingying XIA
Photo ByDepartment of Chemistry, Yan QIU