Recently, Professor Chuang-Chuang Li’s group from the Department of Chemistry at the Southern University of Science and Technology (SUSTech) first reported a type II intramolecular (3+2) dipolar cycloaddition reaction for the syntheses of various bridged bicyclo[m.n.2] ring systems. This method was used as a key step accomplishment of asymmetric total synthesis of natural product nakafuran-8. The computational study was carried out to explore the regioselectivity, chemoselectivity, and diastereoselectivity of type II (3+2) cycloaddition. The study, entitled “Facile generation of bridged medium-sized polycyclic systems by rhodium-catalysed intramolecular (3+2) dipolar cycloadditions,” was published in Nature Communications.

Bridged bicycle ring systems are widely found in natural products with important biological activities, such as medicines Taxol, Picato, and Artemisinin (Figure 1). Due to the unique chemical and biological properties, developing efficient methods that enable the construction of bridged ring systems is highly warranted. Chuang-Chuang Li’s research group has carried out a series of research work focusing on developing new methods for the efficient construction of bridged ring systems and exploring its applications in the total synthesis of natural products. They have achieved many innovative research results, such as the first reported type II [5+2] cycloaddition reaction (Figure 2, Angew. Chem. Int. Ed., 2014, 53, 11051; Angew. Chem. Int. Ed., 2015, 54, 1745).

Figure 1. Natural products with Bridged ring systems
Several great synthetic challenge nature products were total synthesis using type II [5+2] cycloaddition reaction as a key step, including the first total synthesis of challenging cyclocrinol and cerorubenic acid III and the efficient asymmetric total synthesis of star molecule vinigrol (J. Am. Chem. Soc., 2018, 140, 5365; J. Am. Chem. Soc., 2019, 141, 2872; J. Am. Chem. Soc., 2019, 141, 15773.). Now, type II [5+2] cycloaddition reaction is a very effective strategy for constructing bridged bicyclo[m.n.1] ring systems (Acc. Chem. Res., 2020, 53, 703.). In contrast to the approaches for the creation of bicyclo[m.n.1] ring systems, bridged bicyclo[m.n.2] ring systems pose a great synthetic challenge, and chemistry for bicyclo[m.n.2] ring systems remain underdeveloped.

Figure 2. Type II [5 + 2] cycloaddition reaction and its application in the synthesis of bridged natural products
In this work, Professor Chuang-Chuang Li’s research group first reported the type II (3 + 2) cycloaddition reaction (Figure 3) for constructing bridged bicyclo [m.n.2] ring systems. A series of [m.n.2] bridge ring system products were efficiently synthesized with good design, including highly challenging bridged medium-sized 8-membered ring and 9-membered ring.
Notably, this cycloaddition constructed two carbocyclic rings and one heterocyclic ring, and three new stereogenic centers, including one bridgehead all-carbon quaternary stereogenic center in a single step. Next, a key step in the asymmetric total synthesis of nakafuran-8 was accomplished using type II (3+2) cycloaddition reaction. Finally, quantum mechanical calculations were carried out with density functional theory and solvation energies computed at the ωB97X-D/6-311 + G(d,p), CPCM(DCE) level of theory to explore the regioselectivity, chemoselectivity, and diastereoselectivity of the (3+2) cycloaddition.
This type II (3+2) reaction provides new access to the bridged ring system’s efficient synthesis and lays the foundation for further bridged ring system research and new drugs development.
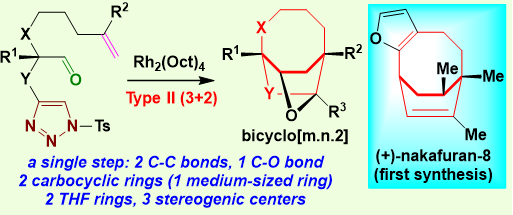
Figure 3. Type II (3+2) cycloaddition reaction and asymmetric total synthesis of nakafuran-8
Dr. Bao-Long Hou, Ms. Na Lv, and Mr. Yong-Qiang Wang from the Department of Chemistry at SUSTech are the first co-authors of this paper. Prof. Chuang-Chuang Li from the Department of Chemistry at SUSTech and K. N. Houk from the University of California are the co-corresponding authors.
This work was supported by the National Natural Science Foundation of China (NSFC), Guangdong Provincial Key Laboratory of Catalysis, Research Projects of Universities of Guangdong Province, and the Shenzhen Peacock Plan.
Paper link: https://www.nature.com/articles/s41467-021-25513-7
Proofread ByAdrian Cremin, Yingying XIA
Photo By