Insulin is the primary hormone controlling blood glucose levels. Altered insulin secretion is frequently found in diabetes patients. Understanding mechanisms regulating insulin secretion is therefore of central importance to biology and medicine.
Beta cells, the insulin-secreting cells inside the pancreas, are regulated by a combination of factors, including nutrients, hormones, and the nervous system. Among these, neural regulation of beta cells remains understudied.
Finding the “Turtle” messenger for signals
Researchers at the Southern University of Science and Technology (SUSTech) have discovered a new messenger of G-protein coupled receptors (GPCRs) that controls insulin secretion and glucose clearance in response to central stimulation of the brain. The messenger, 5-IP7, is structurally similar to a turtle (Figure 1) and is generated upon stimulation of the muscarinic receptor M3R by the neurotransmitter acetylcholine, which in turn is released at the parasympathetic nerve terminal innervating pancreatic β-cells.
Associate Professor Feng Rao’s group from the School of Life Sciences at SUSTech, in collaboration with Professor Chao Wang’s group at the University of Science and Technology of China (USTC), published a research article in Nature Metabolism entitled “5-IP7 is a GPCR messenger mediating neural control of synaptotagmin-dependent insulin exocytosis and glucose homeostasis.” The paper discovered 5-IP7 as a GPCR messenger that works together with Ca2+ to specifically mediate neural stimulation of insulin secretion.
Professor Rao’s lab focused on delineating the emerging signaling role of a family of endogenous metabolites known as inositol polyphosphates (IPs). Over the past two years, the same group discovered IP6 as a metabolic regulator of the Cullin RING E3 Ligases (CRL) during the process of glucose-induced insulin secretion (PNAS, 2020; Nature Communications, 2021).
How does the brain generate the signals to insulin secretion?
The researchers started by learning how IP6K1, a 5-IP7 synthase, is regulated, which leads to the delineation of a signaling pathway that mediates IP6K1 phosphorylation and activation by the neurotransmitter acetylcholine. The team then generated transgenic mice carrying a hyperactive IP6K1 mutant that mimics acetylcholine-induced phosphorylation. Functional characterization of these mutant mice revealed an essential role of 5-IP7 in promoting insulin secretion. Employing a couple of approaches to stimulate the central brain, the team finally concluded that brain stimulation releases acetylcholine at the nerve terminal to activate IP6K1 in β-cells, generating 5-IP7 to signal insulin secretion.
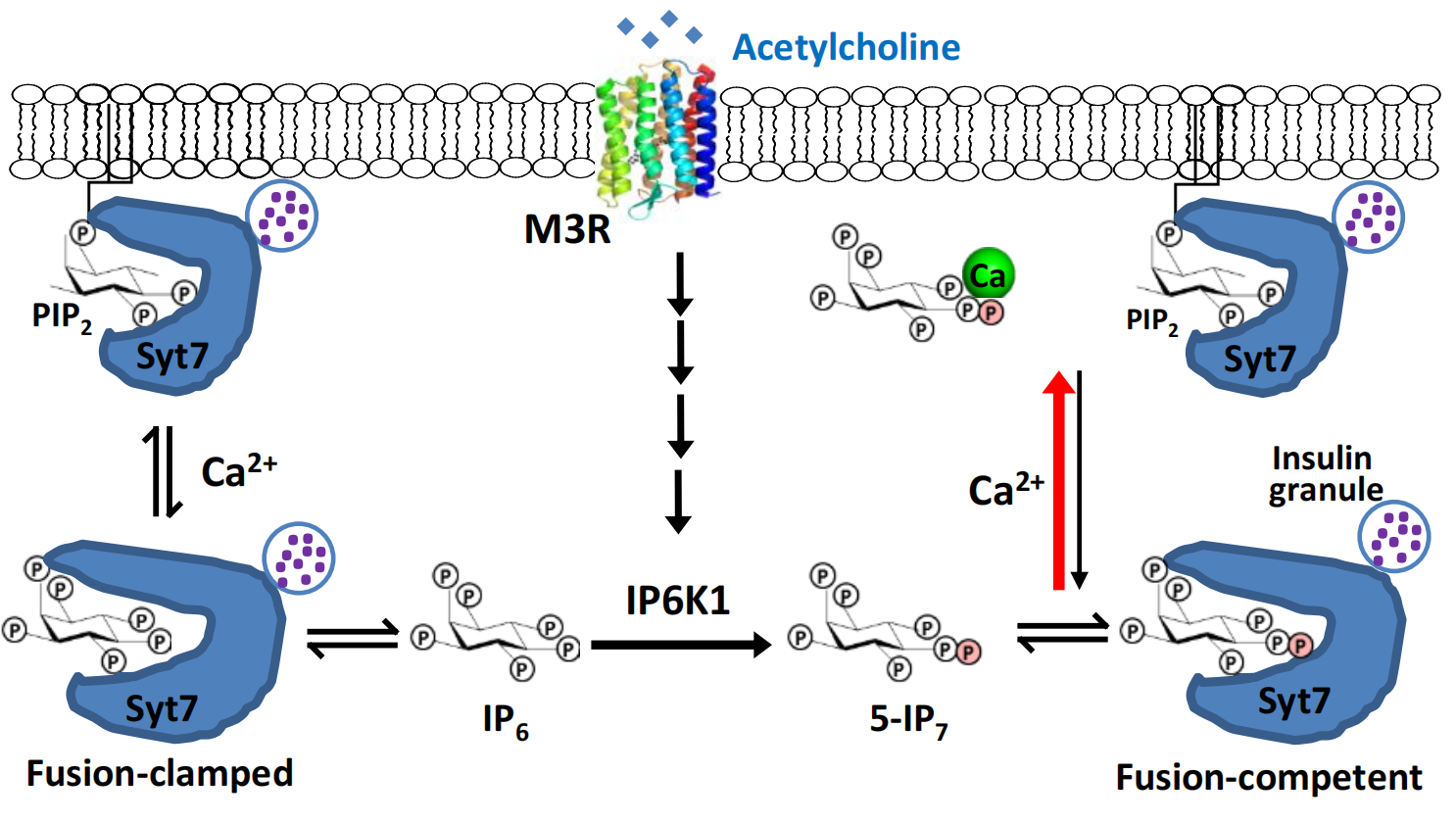
Figure 1. Schematic diagram illustrating the proposed role of 5-IP7 and Ca2+ as coincident messengers sensing the neurotransmitter acetylcholine to promote Syt7-PIP2 interaction. Compared to the non-specific IP6-Ca2+ interaction, the 5’-β-phosphate augments 5-IP7-Ca2+ binding, thereby sensitizing Syt7 for Ca2+-dependent membrane interaction.
A new paradigm to understand insulin vesicle release control
Next, to identify the mechanistic target of 5-IP7, the team showed that 5-IP7 does not affect insulin biogenesis. Instead, 5-IP7 binds to a protein called Synaptotagmin 7 (Syt7), a known Ca2+ sensor to trigger insulin vesicle release. In the absence of Ca2+, 5-IP7-Syt7 interaction prevents Syt7 binding to the plasma membrane lipid PIP2. Ca2+-influx leads to the formation of a high-affinity Ca2+-5-IP7 complex, which enables Syt7-PIP2 interaction and insulin vesicle-plasma membrane fusion.
The research uncovered for the first time a GPCR messenger role for 5-IP7. Moreover, 5-IP7 and Ca2+ are proposed to act as coincident messengers during the secretion of insulin and perhaps other types of vesicles. Therefore, the study set a new conceptual paradigm to understand signal transduction mediated by the ubiquitously present but relatively poorly understood metabolites 5-IP7.
Given that GPCRs are already the focus of active pharmaceutical research and development to treat many diseases, including type II diabetes, a similar evaluation of targeting 5-IP7 metabolism is advocated in diseases mediated by Gq-linked GPCRs.
This work was primarily conducted in the laboratory of Associate Professor Feng Rao and Professor Chao Wang, who are also the corresponding authors. The five co-first authors are Ph.D. students Xiaozhe Zhang and Na Li, master’s student Jun Zhang, Research Assistant Xiaoli Yang from Professor Rao’s lab, and Ph.D. student Yanshen Zhang from Professor Wang’s lab.
This research was supported by the National Science Foundation of China (NSFC), the Shenzhen Municipal Science and Technology Innovation Commission, and the Guangdong Provincial Department of Science & Technology.
Paper link: https://www.nature.com/articles/s42255-021-00468-7
Previous research paper (PNAS, 2020): https://www.pnas.org/content/117/8/4117.short
Previous research paper (PNAS, 2021): https://www.nature.com/articles/s41467-021-22941-3
Professor Feng Rao’s webpage: https://faculty.sustech.edu.cn/raof/en/
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo BySchool of Life Sciences