Alkylated aromatics play an important role in medicinal chemistry, materials chemistry, and energy chemistry. Direct alkylation of the C-H bonds of arenes in a selective manner is one of the most important and straightforward ways to synthesize alkylated aromatics.
Classical methods for direct alkylation of arenes include the Friedel-Crafts alkylation reaction of arenes, the Transition-metal-catalyzed ortho-alkylations of C-H of arenes in the assistance of directing groups, and the Transition-metal- or visible-light-catalyzed C-H alkylations of arenes using diazo esters as specific alkylation reagents. However, the known methods suffer from the use of transition metals, limited functional group tolerance and scope, as well as harsh conditions. Therefore, developing direct alkylation of the C-H bond of arenes under mild conditions is highly desirable yet challenging.

Associate Professor Wei Shu’s research team of the Department of Chemistry at the Southern University of Science and Technology (SUSTech) has recently made significant progress toward visible-light-catalyzed regioselective C-H alkylation of arenes using alkenes as the alkylating reagents.
Their research, entitled “Organophotocatalytic Regioselective C-H Alkylation of Electron-Rich Arenes Using Activated and Unactivated Alkenes,” was published online in Angewandte Chemie International Edition, one of the prime chemistry journals in the world.
Prof. Shu’s group developed an efficient method for visible-light-catalyzed direct C-H alkylation of the C-H bond of arenes using olefins as alkylating reagents at room temperature. The metal-free conditions enable the regioselective alkylation of the C-H bond of arenes in one step from readily-available starting materials. This method circumvents the pre-functionalization of substrates and features a 100% atom economy (Figure 1).
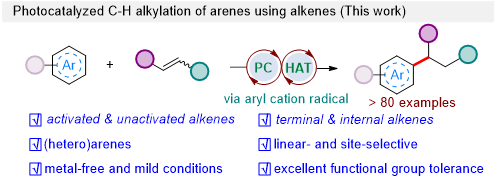
Figure 1. Photocatalyzed C-H alkylation of arenes using alkenes
Under optimized conditions, a wide range of aromatic and hetero-aromatic compounds such as thiophene, furan, pyrrole, pyridine, and benzene can undergo regioselective C-H alkylation reactions with alkenes. Moreover, styrenes and aliphatic alkenes, as well as terminal and internal alkenes, can be used as alkylating reagents to achieve selective C-H alkylation of aromatics (Figure 2).
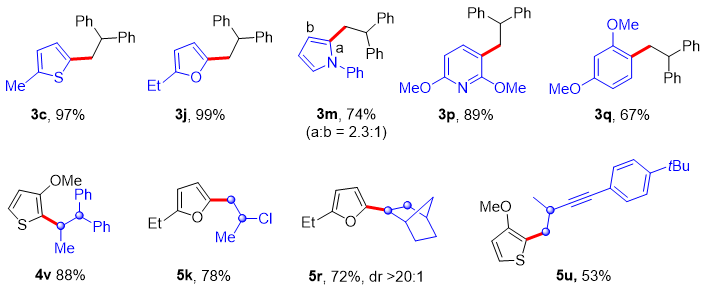
Figure 2. Selected examples for regioselective C-H alkylation
The above-mentioned research was conducted at SUSTech. Graduate student Bi-Hong Chen is the first author of this paper. Prof. Wei Shu is the corresponding author. Profs. Feng He and You-Yun Zhou of the Department of Chemistry also assisted in the photo- and electrochemical property measurements.
This work was supported by the National Natural Science Foundation of China (NSFC), National Natural Science Foundation of Guangdong, Department of Education of Guangdong Province, Pearl River Talent Recruitment Program, Guangdong Provincial Key Laboratory of Catalysis, and the Stable Support Plan Program of Shenzhen Natural Science Fund. The researchers also acknowledge the assistance of SUSTech Core Research Facilities.
Paper link: https://onlinelibrary.wiley.com/doi/10.1002/anie.202200773
To read all stories about SUSTech science, subscribe to the monthly SUSTech Newsletter.
Proofread ByAdrian Cremin, Yingying XIA
Photo By